Follow us on Google News (click on ☆)
In a paper published in Proceedings of the National Academy of Sciences, scientists, after overcoming several technological barriers, were able to visualize in real time the transfer of antibiotic resistance DNA within these biofilms. They demonstrate the importance of the shape and spatial organization of these communities in the spread of resistance.
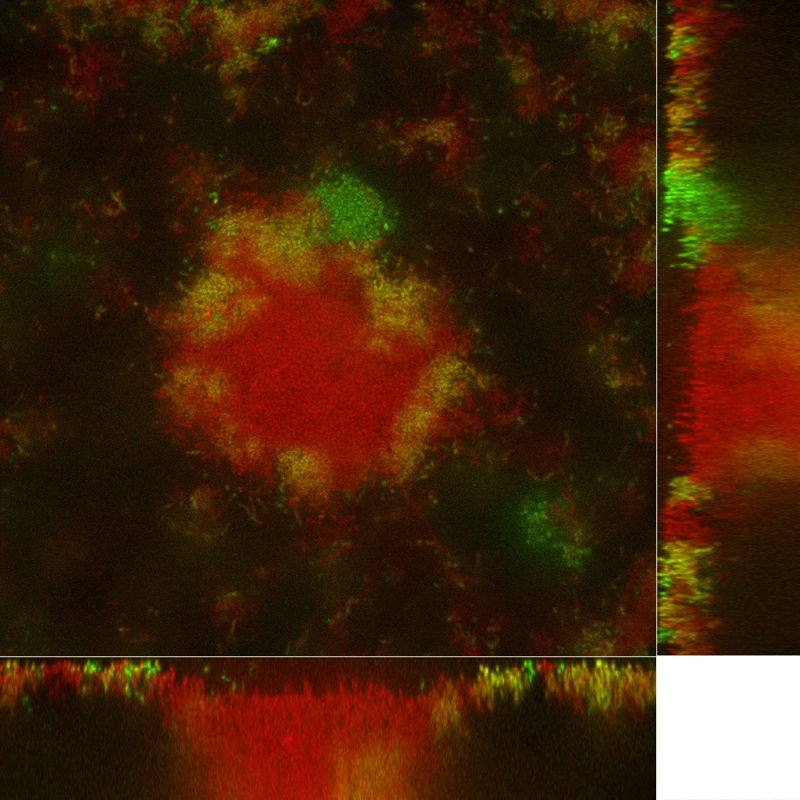
Figure: Image of a bacterial biofilm.
Escherichia coli obtained by confocal microscopy.
The green bacteria carry an antibiotic resistance plasmid. This plasmid can be transferred to the red bacteria, which then turn yellow by producing both green and red fluorescence. The fluorescence allows identification of bacteria that have acquired resistance. The side views show vertical sections of the biofilm; the main image is a top view.
© Knut Drescher
Biofilms are groups of bacteria that live together as aggregates. Within these biofilms, bacteria can exchange DNA elements called plasmids. These plasmids can contain special genes that allow bacteria to become resistant to antibiotics. This means that even if antibiotics are used to try to kill these bacteria, those with these genes can survive.
Bacteria in biofilms can retain these plasmids and thus maintain their ability to resist antibiotics for later. It's somewhat like keeping a secret weapon, just in case they need it.
But what about their potential to acquire these resistances?
To answer this question, it is essential to be able to track plasmid transfer between biofilm bacteria in real time. Several challenges remained:
- The complexity of biofilms with their three-dimensional structure makes direct observation of bacterial interactions difficult.
- The tools and technologies needed to observe these exchanges in real time and at microscopic scale are not yet sufficiently developed.
- Plasmid exchanges can be rapid and sporadic, making them difficult to capture with traditional methods.
Filming plasmid exchanges within biofilms in real time
In a paper published in the journal PNAS, scientists, by developing innovative new genetic and microscopy tools, succeeded in visualizing DNA transfer within bacterial communities in real time. They were thus able to film the resistance acquisition process by bacteria organized in structured biofilms. These initial results show that when the biofilm becomes dense and reaches maturity, its physical configuration limits the necessary contacts between donor and recipient bacteria, slowing plasmid diffusion. Thus, the very architecture of biofilms plays a decisive role in the transmission and acquisition of antibiotic resistance.
This study therefore shows that while biofilms promote plasmid maintenance once resistance is acquired, they would be less favorable for acquiring new resistances. Their structure could instead actively limit the initial acquisition of these genetic elements.