🎯 Cancer: this innovative device eliminates resistant tumors
Follow us on Google News (click on ☆)
The TAR-200 device comes in the form of a small pretzel-shaped implant that gradually releases the chemotherapy drug gemcitabine directly into the bladder. Unlike traditional instillations where the liquid drug remains for only a few hours, this system maintains a constant therapeutic concentration for three full weeks per treatment cycle. This prolonged exposure time allows the active ingredient to penetrate deeper into bladder tissues and eliminate cancer cells more effectively.
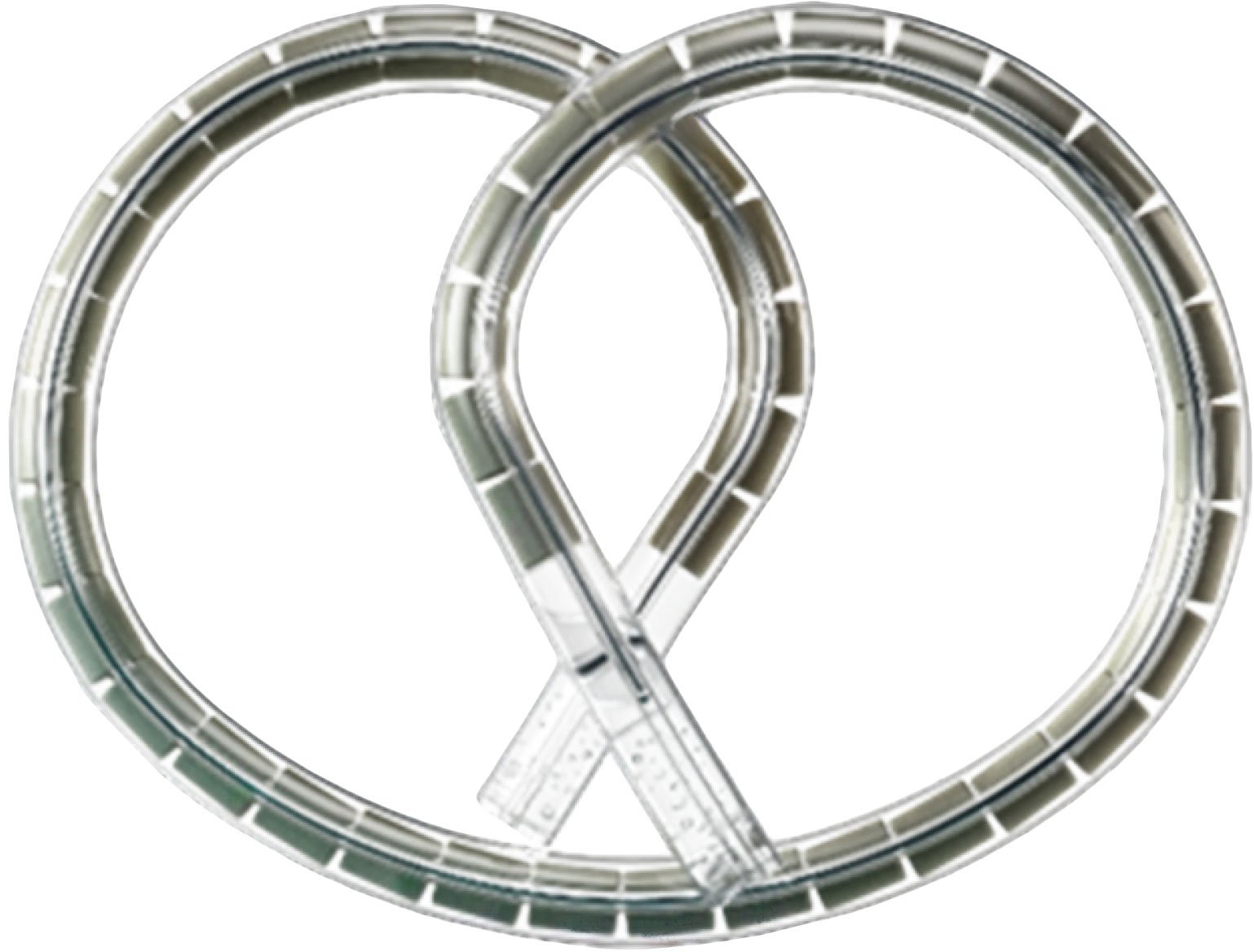
The TAR-200 device, with its particular shape, contains the chemotherapy drug gemcitabine and gradually releases its active ingredient for three weeks per therapeutic cycle.
Credit: Johnson & Johnson
The international SunRISe-1 clinical trial included 85 patients with high-risk non-invasive bladder cancer who had not responded to standard BCG treatment. These patients were traditionally candidates for radical cystectomy, a surgical removal of the bladder involving significant risks and major consequences on quality of life. The treatment protocol consisted of TAR-200 administrations every three weeks for six months, then four times per year during the following two years.
The results published in the Journal of Clinical Oncology reveal that 70 out of 85 patients saw their cancer completely disappear within three months following treatment. Nearly half of these patients maintained this complete remission after one year of follow-up. The treatment proved well-tolerated with minimal side effects, unlike the combination with other immunotherapies which proved less effective and more toxic.
Doctor Sia Daneshmand, principal investigator of the study, emphasizes that this approach represents the most effective therapeutic option ever documented for this common form of bladder cancer. The US Food and Drug Administration has granted TAR-200 an accelerated review procedure, recognizing its significant therapeutic potential. Several additional clinical trials are currently exploring the expanded applications of this extended-release technology.
This therapeutic innovation opens promising perspectives for localized cancer treatment, where prolonged drug delivery could revolutionize the management of many tumor pathologies. The ability to maintain an optimal therapeutic concentration directly at the tumor site while minimizing systemic effects represents a considerable advantage compared to conventional approaches.
High-risk non-invasive bladder cancer
Non-invasive bladder cancer represents the most frequent form of this pathology, developing in the inner wall of the organ without reaching the deeper muscle layers. This superficial location theoretically allows for localized treatment, but certain factors like tumor size, their number, or their aggressive nature define the high risk of recurrence.
Classification as high risk implies that these tumors present a significant probability of reappearing after initial treatment, or even progression toward more invasive forms. Affected patients require close monitoring and more intensive treatments than those with low-risk forms.
The standard treatment for these cases consists of intravesical BCG instillation, an immunomodulator derived from an attenuated bacterial strain. However, approximately 30 to 40% of patients do not respond adequately to this therapy, creating a therapeutic impasse requiring alternative approaches.
BCG treatment failure traditionally leads to proposing radical cystectomy, a major surgical intervention involving complete removal of the bladder with urinary reconstruction, associated with significant morbidities and a profound impact on patients' quality of life.
Extended-release drug delivery systems
Extended-release drug delivery systems represent an innovative therapeutic approach allowing maintenance of a constant concentration of active ingredient at the action site. Unlike traditional administrations that create peaks and troughs in concentration, these devices release the medication in a controlled manner over an extended period.
In the oncological field, this technology presents the major advantage of continuously exposing tumor cells to optimal therapeutic doses, thereby increasing treatment effectiveness while reducing administration frequency. Extended release also allows reaching tumor areas less accessible to conventional treatments.
The release mechanism can rely on different physicochemical principles: diffusion through a polymer membrane, gradual erosion of the device, or controlled osmotic systems. Each technology is optimized according to the medication characteristics and therapeutic application site.
These systems open therapeutic perspectives in many fields beyond oncology, particularly for chronic diseases requiring continuous treatment, localized infections, or inflammatory pathologies where maintaining a stable concentration significantly improves treatment effectiveness.