⚛️ Major breakthrough: germanium used everywhere in electronics becomes superconducting
Follow us on Google News (click on ☆)
Semiconductors like germanium and silicon are fundamental elements of our daily technology, present in computer chips and optical fibers. Their particular crystalline structure, similar to that of diamond, gives them electronic properties intermediate between metals and insulators. This versatility makes them materials of choice for the electronics industry, but making them reach the superconducting state requires structural modifications at the atomic level.
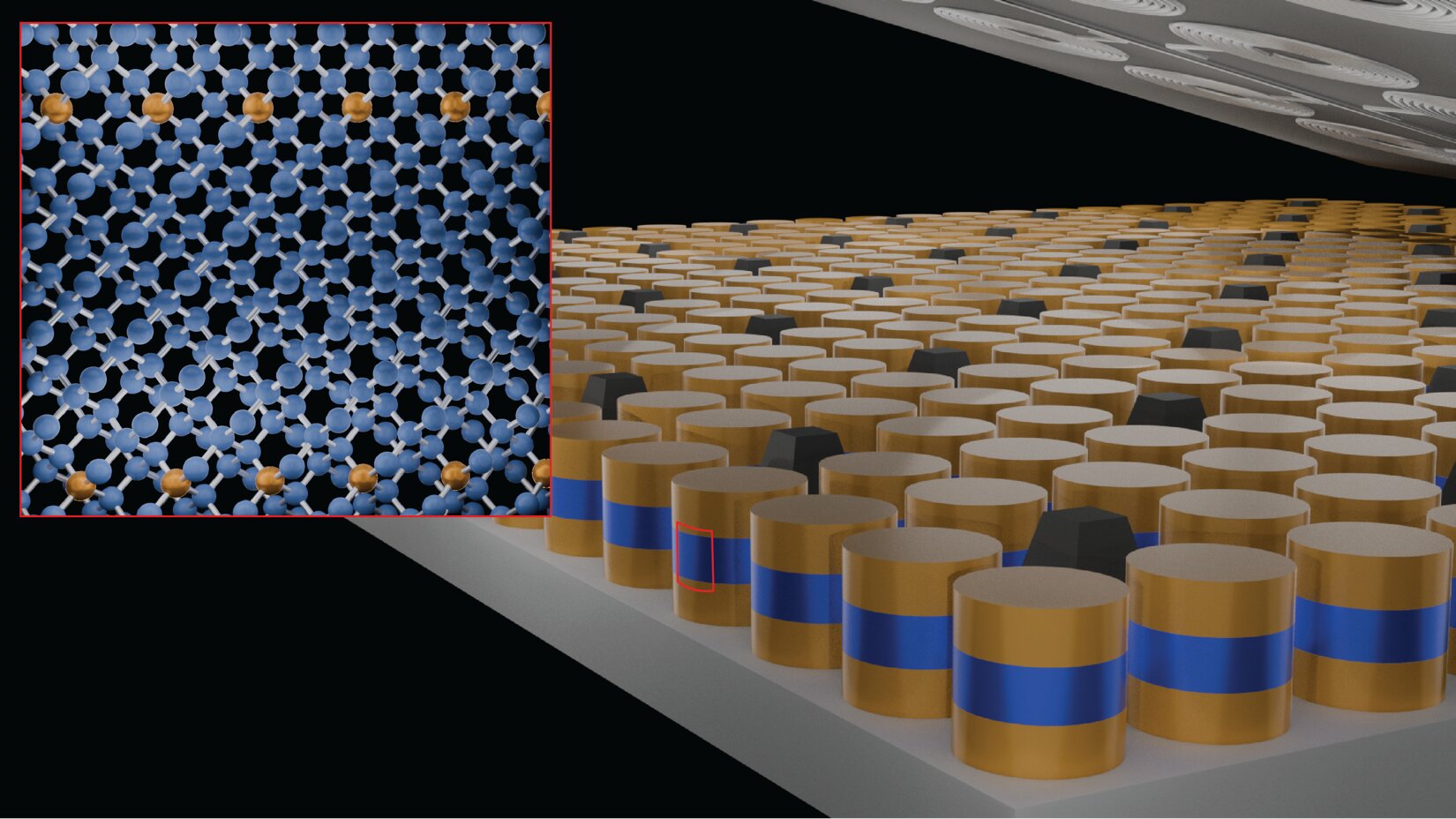
Josephson junction structures using different forms of germanium: super-Ge (in gold), semiconductor Ge (in blue) and super-Ge. The inset shows the crystalline form of Super-Ge.
Credit: Patrick Strohbeen / NYU
The international team developed an innovative approach using a high-precision crystal growth technique. This method allows for the incorporation of gallium atoms into the germanium crystal lattice at exceptionally high concentrations. Unlike traditional doping methods that often lead to structural instability, this approach maintains crystal integrity while inducing superconductivity.
The process results in a controlled deformation of the crystalline structure that allows electrons to form pairs and move without any electrical resistance. This superconductivity manifests at a temperature of 3.5 Kelvin, or approximately -269 degrees Celsius (-452°F). The structural stability achieved allows for the consideration of large-scale industrial applications, particularly in the fabrication of quantum circuits.
Since germanium is already a well-mastered material in the semiconductor industry, this breakthrough could facilitate the integration of superconducting components into existing technologies without requiring a complete overhaul of manufacturing processes.
This research demonstrates how precise modification of crystalline structures can reveal new physical properties in materials that are otherwise well-known. Scientists now plan to explore other element combinations and refine growth techniques to achieve higher superconducting temperatures, which would significantly expand the field of practical applications.
Superconductivity explained
Superconductivity is a special quantum state where certain materials conduct electricity without any resistance. This phenomenon generally occurs at very low temperatures and allows electric current to circulate indefinitely without energy loss.
In an ordinary conductor, electrons move while encountering obstacles in the crystal lattice, which generates heat and energy dissipation. In contrast, in a superconductor, electrons form pairs called Cooper pairs that move in a coordinated manner through the material.
These electron pairs interact with the vibrations of the crystal lattice (phonons) in a way that allows them to bypass all potential obstacles. The result is a perfectly efficient electrical flow that encounters no opposition, somewhat as if electrons were sliding on a perfectly smooth surface without friction.
The critical temperature is an essential parameter: it's the maximum temperature below which the material becomes superconducting. For most conventional superconductors, this temperature is extremely low, requiring cooling with liquid helium, which still limits practical applications.
Semiconductor doping
Doping is a fundamental technique in materials science that involves deliberately introducing foreign atoms into a semiconductor crystal to modify its electrical properties. These added atoms, called impurities, can either provide additional electrons or create 'holes' where electrons are missing.
In the case of gallium-doped germanium, gallium atoms replace some germanium atoms in the crystal lattice. Gallium has one less valence electron than germanium, which creates positively charged 'holes' in the electronic structure. These holes can move and conduct electric current.
When doping reaches very high levels, it's called hyperdoping. This exceptional concentration of foreign atoms profoundly modifies the electronic behavior of the material. Instead of simply increasing conductivity, it can induce spectacular phase transitions, such as the transition to the superconducting state.
The major difficulty lies in maintaining the structural stability of the crystal despite the massive introduction of foreign atoms. Modern epitaxy techniques allow for precise atomic control of this process, avoiding the collapse of the crystal structure while enabling the desired electronic modifications.