Follow us on Google News (click on ☆)

Illustration image Pixabay
The technique employed combines high pressure and high temperature to produce small discs of this ultra-hard diamond. Published in Nature, these results could revolutionize fields such as electronics or drilling tool manufacturing. Traditional diamonds, known for their hardness, could thus be replaced.
The molecular structure of ordinary diamonds is based on carbon atoms forming perfect tetrahedrons. This organization, called face-centered cubic, is what gives diamond its exceptional resistance. Lonsdaleite, on the other hand, has a hexagonal structure, with layers of carbon arranged differently.
Wenge Yang's team reproduced the extreme conditions of a meteorite impact in the laboratory to synthesize this hexagonal diamond. By compressing purified graphite at pressures equivalent to 200,000 atmospheres and heating it with a laser, they obtained this promising material. Despite the presence of impurities, analyses confirmed the hexagonal structure.
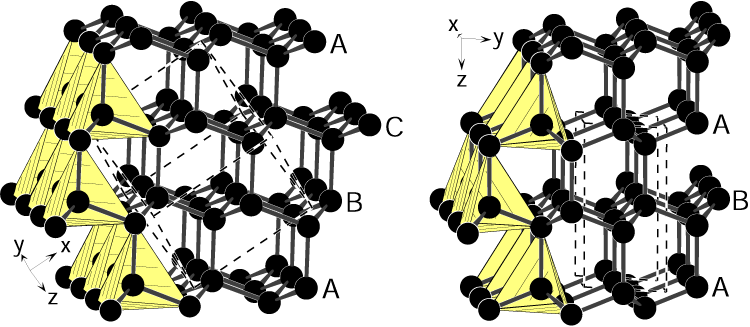
Structural differences between cubic diamond (left) and hexagonal diamond (right).
Credit: Ralf Riedel
The potential applications of lonsdaleite are vast, ranging from high-performance electronics to quantum technologies. However, the production of larger and purer crystals is necessary to fully exploit its properties. Researchers estimate that its industrial adoption could take about ten years.
What is lonsdaleite?
Lonsdaleite is a form of diamond with a hexagonal crystal structure, different from the structure of ordinary diamonds. It was initially identified in meteorites, where it forms under the effect of extreme pressures and temperatures.
This diamond variant is theoretically harder than classic cubic diamond, thanks to its unique atomic arrangement. Carbon atoms are arranged in alternating layers, which could offer increased resistance to deformation.
The synthesis of lonsdaleite in the laboratory represents a significant advance. It allows studying its properties without depending on rare meteorite samples, opening the way to industrial applications.
Despite this progress, challenges remain, such as producing pure crystals of sufficient size. These obstacles must be overcome for lonsdaleite to be used on a large scale.
How is lonsdaleite synthesized?
The synthesis of lonsdaleite requires extreme conditions, similar to those encountered during a meteorite impact. Researchers use a diamond anvil cell to generate colossal pressures, combined with laser heating.
The process begins with purified graphite, a less dense form of carbon. Under pressure, the graphite layers rearrange to form the characteristic hexagonal structure of lonsdaleite.
Mastering these parameters is crucial to avoid the formation of classic cubic diamond or impurities. Researchers carefully adjust pressure and temperature to favor lonsdaleite formation.
This synthesis method offers precise control over material formation. It constitutes a step toward the production of lonsdaleite for practical applications.