Follow us on Google News (click on ☆)

Illustration image Pixabay
Since the 1953 Miller experiment, which demonstrated that chemical reactions could give rise to life on Earth, part of the research has focused on discovering potential synthesis pathways under the conditions of the Earth's early environment. Other scientists have instead turned their attention to understanding what constitutes life itself.
Robert Pascal, emeritus research director at the Laboratory of Physics of Ionic and Molecular Interactions (CNRS/Aix-Marseille Univ.), has proposed a new minimalist theoretical model of living processes. In this system, based on simple autocatalytic cycles, an energy input compensates for thermodynamic instability and allows kinetic stability to be achieved through the replication of the initial activated molecule.
This model is based on a thermodynamically activated molecule that, when supplied with energy, transforms into a second compound, which can then possibly transform into a third compound. After a complete cycle, the process produces two copies of the original molecule, enabling the cycle to continue.
The system is thus capable of growing and reproducing. In the absence of energy, the degradation of the activated species leads to byproducts that cannot sustain the cycle: the system "dies" permanently and cannot be restarted.
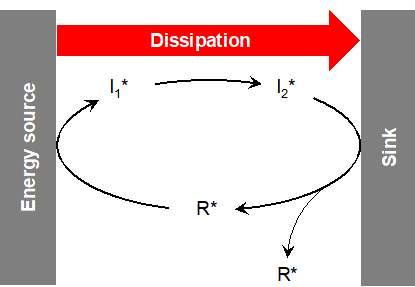
The base molecule, R, is activated into R* through energy input. R* creates a first activated intermediate, I1*, which produces a second intermediate: I2*. The decomposition of I2* generates at least two copies of R*, enabling the cycle to grow and restart. The molecules are continuously renewed, but the system remains stable.
© Robert Pascal
This theoretical model also takes into account other aspects of life, such as natural selection. Multiple different systems can compete for the same energy source, allowing the most efficient one to prevail while the others perish. While it does not include the adaptability and variation of a genetic code, such a system might have emerged before this principle came into play.
Thus, the model recreates the behavior of living organisms, which are capable of emergence, growth, competition with their peers, and death. This model also has the advantage of being able to start from just one extremely rare and unstable chemical species within a molecular jumble. Future work will focus on identifying chemical compounds that are compatible with Earth's early environment and capable of reproducing this theoretical model.
Editor: MK
References:
R. Pascal. Evolutionary Abilities of Minimalistic Physicochemical Models of Life Processes.
Chem. Eur. J. 2024, 30, e20241780.
https://doi.org/10.1002/chem.202401780
https://exobiologie.fr/emergence-de-la-vie-vers-des-modeles-chimiques-minimaux/