A new step in the race for affordable batteries
Follow us on Google News (click on ☆)
These cracks have long affected the performance of such batteries, limiting their lifespan. A team of researchers from Argonne recently developed a method that seems to address this recurring issue.
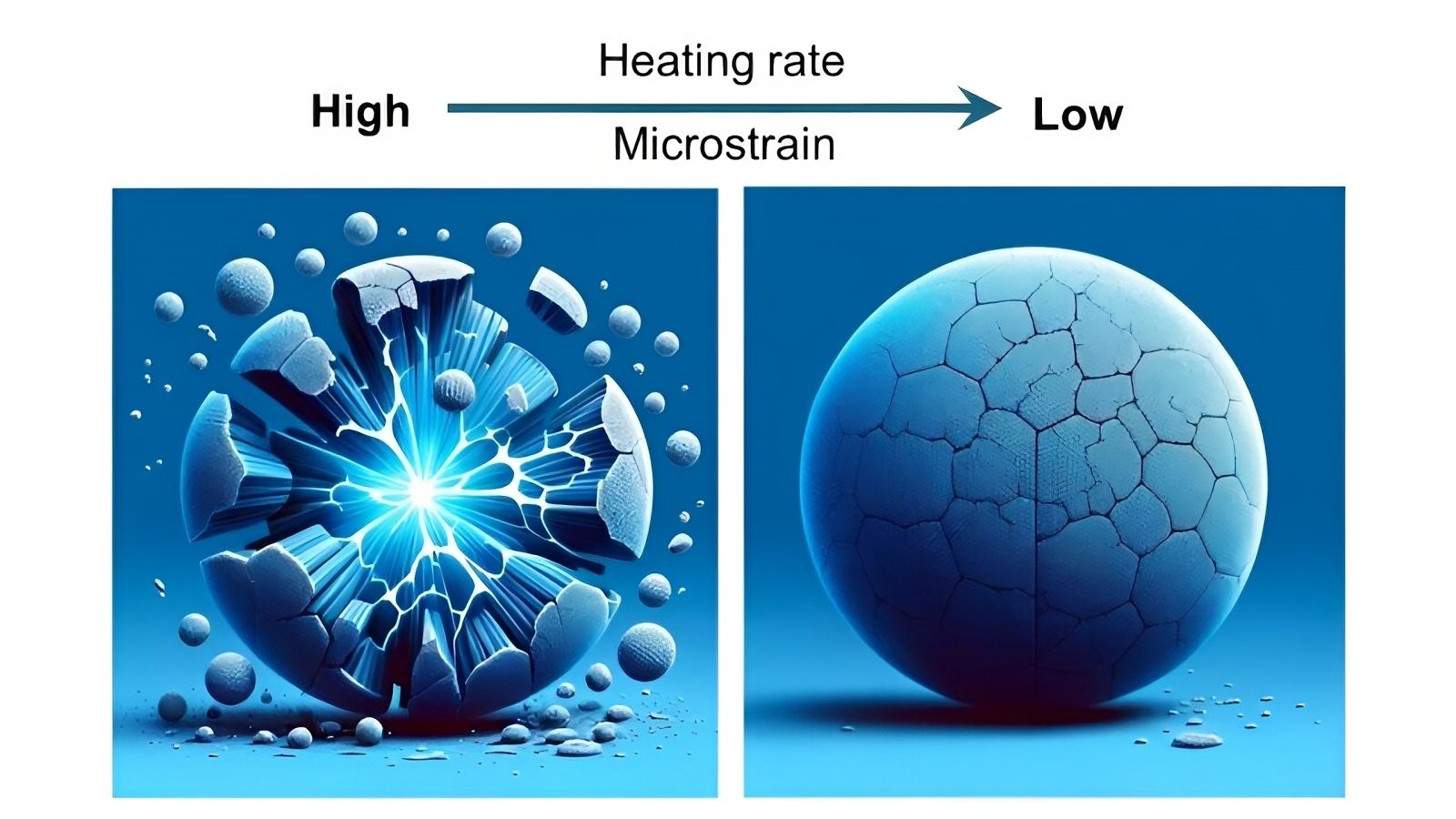
Artistic illustration showing how reducing the heating rate during cathode preparation eliminates cracks.
Credit: Argonne National Laboratory.
To understand the cause of these cracks, the scientists focused on the very structure of the cathode particles, which feature a nickel-rich core and a cobalt and manganese shell. This particular configuration allows for high storage capacity, but the imbalance between the materials causes internal stress, generating cracks during charge-discharge cycles.
By adjusting the heating rate during particle preparation, the team discovered that a slower rate helped reduce this stress. The synthesis process, carried out under very precise temperature conditions, stabilized the cathode particles.
The study conducted at Argonne revealed that when the particles were heated at a rate of one degree per minute, cracks did not form. However, a faster heating rate of five degrees per minute led to crack formation starting at 482 °F (250 °C).
This discovery is crucial for the future of sodium-ion batteries. These batteries, less expensive and more abundant than lithium-ion batteries, could play a key role in the energy transition, especially for renewable energy storage.
The researchers continue to improve this technology, aiming to eliminate the use of nickel in cathodes, which would further reduce costs and improve durability. While sodium-ion batteries have a lower energy density than lithium batteries, they show promise for electric vehicles designed for urban use.
The hope is to develop sodium-ion batteries as efficient as current lithium iron phosphate cathodes, which could make electric mobility more sustainable.
What is a sodium-ion battery?
A sodium-ion battery is a type of rechargeable battery that uses sodium ions (Na⁺) to store and release energy, unlike lithium-ion batteries which use lithium ions. Sodium ions move between an anode and cathode during charge and discharge cycles, creating a flow of electrons that generates electricity.
Although lithium-ion batteries dominate the market, sodium is much more abundant and less expensive than lithium. This makes sodium-ion batteries attractive, particularly for applications requiring large-scale energy storage, such as electrical grids. However, these batteries have a lower energy density, meaning they store less energy per unit of weight or volume.