Follow us on Google News (click on ☆)
Enzymes facilitate the chemical reactions that provide energy and transform various components during cellular metabolism. How their properties were acquired throughout evolution is a fundamental question. Indeed, their current functioning is the result of evolutionary steps that occurred over very long periods.
Ancient methanogenic Archaea inhabited a very hot and radioactive environment (D. Mardern / IBS)
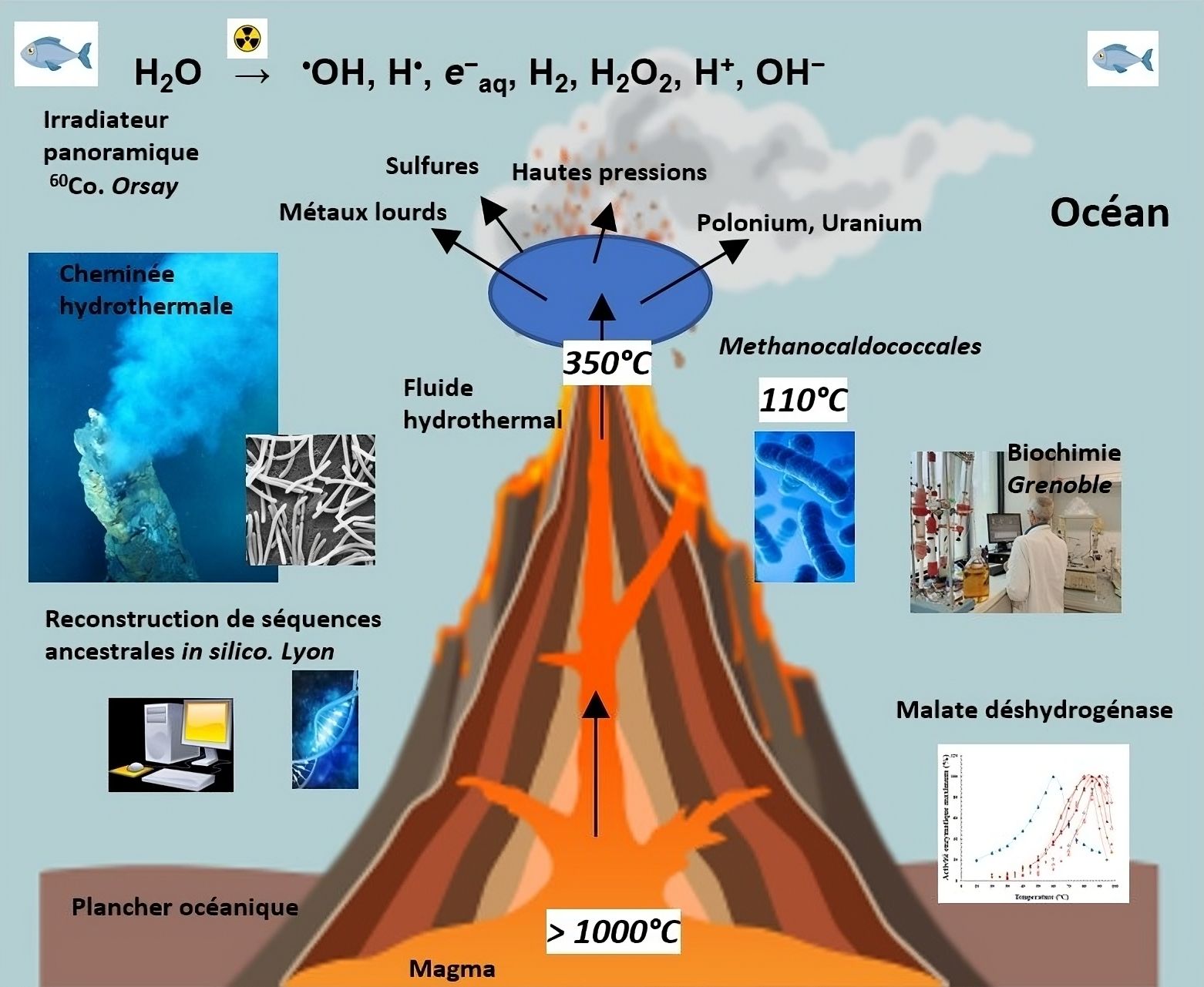
As part of a collaborative project, researchers from Irig/IBS characterized very ancient enzymes, known as extremophiles (capable of functioning under extreme conditions of temperature, pressure, etc.), using a paleo-enzymology approach to understand how some of today's enzymes were constructed.
Certain microorganisms, such as methanogenic archaea (unicellular prokaryotic microorganisms that produce methane), have colonized numerous environments with widely varying temperature conditions. For example, the growth temperature approaches 212°F (100°C) for species isolated from deep hydrothermal vents. Their enzymes are therefore adapted to function under these conditions.
By using malate dehydrogenase, an enzyme involved in metabolism, and applying an evolutionary biochemistry approach coupled with a biophysical approach previously described, which has already proven effective, it was possible to identify the mutations responsible for this enzyme's adaptation within various lineages of modern enzymes from an ancestral form capable of withstanding conditions of radioactivity and temperature considered extremely harmful. Until now, resistance to radioactivity had only been demonstrated at the cellular level, particularly through DNA protection/repair mechanisms.
For the first time, researchers from Irig/IBS demonstrate that there is a favorable capacity for resistance against radioactivity and that this protein property is very ancient.
The discovery of a link between an enzyme's thermal stability and its ability to resist intense radioactive stress opens new perspectives for studying the emergence conditions of ancient cells on an Earth that was more radioactive than today or on other planets. This study also contributes to a better understanding of the rational engineering process of enzymes useful for decontaminating radioactive sites.