Follow us on Google News (click on ☆)
In a study published in Nature Communications, scientists reveal in detail how enzymes that copy DNA participate in repairing this type of damage. In the long term, a deeper understanding of the players involved in this mechanism could allow its manipulation to make cancer cells more sensitive to therapies whose mode of action is to break DNA.
DNA double-strand breaks: a constant threat to our cells.
The DNA that constitutes our genetic heritage is constantly damaged by chemical or physical agents in the environment. One of the most severe damages, the double-strand break, occurs when both strands of the DNA molecule are severed. This lesion can be lethal to a cell if it fails to repair it.
However, human cells have a highly effective repair pathway, non-homologous end joining (NHEJ). This mechanism fuses the broken ends together and can even clean up damaged ends to ensure a perfect fit before sealing them. It was already known that certain enzymes that copy DNA, DNA polymerases, were involved in this adjustment step. But their precise mode of action remained poorly understood.
A key interaction revealed between Ku and X polymerases
In human cells, NHEJ repair begins with the rapid binding of the ring-shaped Ku protein complex to the broken ends. Remarkably, the Ku ring serves as a common anchor point for the various protein complexes that carry out all subsequent steps of NHEJ.
In a study published in the journal Nature Communications, scientists have just elucidated how DNA polymerases from the X family participate in repairing double-strand breaks via NHEJ. This study reveals several atomic-scale structures, obtained by cryo-electron microscopy, showing the interactions between X DNA polymerases and Ku on a DNA break. These structures highlight a specific interaction site between Ku and the BRCT domain, a motif common to all X polymerases.
To prove the importance of this interaction, scientists introduced targeted mutations in the contact area between Ku and the BRCT motif. They then assessed the consequences of these changes on the cells' ability to repair DNA breaks. To do this, they developed an original tool that measures the copying activity at the ends of a DNA molecule broken at a precise point and introduced into cells.
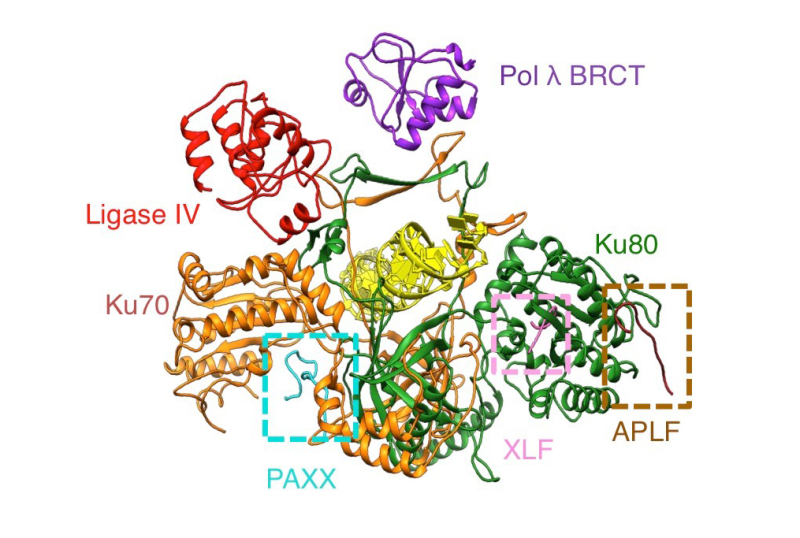
The Ku70/80 ring (orange/green) around the DNA (yellow) serves as an anchor point for the binding of Pol λ (BRCT domain, purple), Ligase IV (BRCT1 domain, red), PAXX (P-KBM domain, cyan), XLF (X-KBM domain, pink), and APLF (A-KBM domain, brown), proteins involved in repairing DNA double-strand breaks via NHEJ.
© Patrick Calsou
The results confirm that the BRCT domain of X DNA polymerases is essential for their recruitment to breaks and their activity, and thus for cell survival in the event of a double-strand break.
A step toward more targeted cancer therapies.
Treatments such as radiotherapy and other cancer therapies aim precisely to induce numerous double-strand breaks in tumor cells to trigger lethal stress. A better understanding of the NHEJ mechanism and all its players paves the way for more precise therapeutic strategies, such as selectively weakening cancer cells by disrupting their ability to repair DNA.