Follow us on Google News (click on ☆)
Atoms, the fundamental building blocks of matter, are primarily composed of empty space. The extremely small nucleus is surrounded by moving electrons. Despite this overwhelmingly empty structure, solid objects resist penetration due to fundamental forces.

Electrostatic repulsion prevents atoms from approaching each other. The negatively charged electrons surrounding each atomic nucleus repel each other when they come close. This phenomenon is similar to the repulsion between two magnets of the same poles. It maintains the solidity of materials by preventing any atomic overlap.
The Pauli exclusion principle reinforces this impossibility. Electrons, being fermions, cannot occupy the same quantum state. Even if we imagine two electron clouds overlapping, this principle forbids such configuration. Thus, even under pressure, atoms don't merge, preserving the integrity of matter.
Yet, quantum mechanics introduces a theoretical exception with the tunnel effect. A particle can cross an energy barrier with a minuscule probability. For a macroscopic object like a human, with so many atoms and particles, this probability is practically zero. It's so low that it wouldn't occur within the age of the Universe.
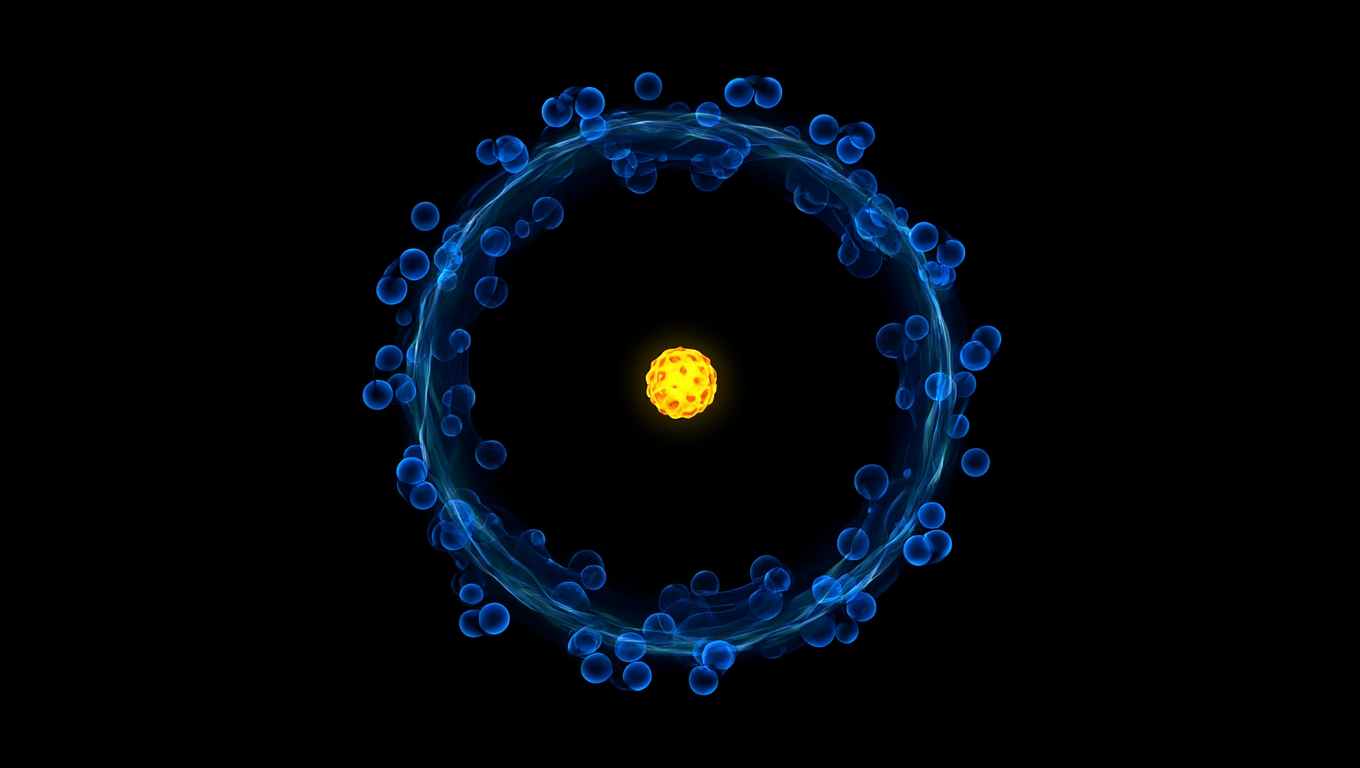
In the atom, between the nucleus and the electron cloud, there is empty space, lots of empty space.
These principles explain why we cannot walk through walls. They ensure the stability of our everyday world. Without them, matter wouldn't have a defined shape, and the Universe would be radically different.
What is electrostatic repulsion?
Electrostatic repulsion is a force that pushes particles with the same charge away from each other. It stems from Coulomb's law in electromagnetism.
This force acts at a distance and depends on the magnitude of the charges and their separation. It gives rise to numerous properties, such as the structure of atoms and molecules. For solid materials, it prevents atoms from being compressed beyond a certain limit. It thus contributes to the rigidity and hardness of objects.
Understanding this force helps explain why microscopic-scale interactions dictate macroscopic behavior.
How does quantum tunneling work?
Quantum tunneling is a quantum phenomenon where a particle crosses an energy barrier that it shouldn't be able to overcome classically. It results from the wave nature of particles.
The particle's wave function doesn't vanish instantly at the barrier but decreases exponentially. If the barrier is sufficiently thin, there's a non-zero probability of finding the particle on the other side.
Although counterintuitive, it's well established experimentally and has important technological applications.