Follow us on Google News (click on ☆)
Whether present in a cappuccino, shaving foam or a fire extinguisher, foams are part of our daily lives. Unfortunately, composed of liquid and gas, they are intrinsically unstable. On Earth, they thus evolve rapidly due to liquid drainage. The most stable foams survive this first evolution, but even once most of the drainage is complete, their bubbles continue to grow, either by merging with each other or through a mechanism called coarsening: small bubbles shrink and disappear while larger ones grow, with gas diffusing from one to another through the liquid phase.

Pixabay illustration image
These later stages of evolution depend on rather subtle physicochemical characteristics of the mixture constituting the foaming liquid, which explains why even today, the general prediction of the stability time of a given foam based on its composition remains an open question.
Yet understanding and slowing down these phenomena is essential for improving the performance of industrial foams. An obstacle to this progress comes from the fact that on the ground, drainage due to gravity disrupts measurements by creating an inhomogeneous liquid distribution.
To observe coarsening without interference, a consortium of researchers conducted observations of foam evolution generated aboard the International Space Station, an environment with effectively zero gravity. They were thus able to compare the stability of very liquid foams (called "wet foams") prepared with nine different stabilizing agents - conventional surfactants, a polymer and a food protein. To do this, they measured the bubble growth rate extremely precisely and showed that it varies by a factor of 10 depending on the chemical nature of the stabilizer used.
Until now, specialists in the field thought that this speed depended mainly on the permeability of the interstitial liquid (that is, the ability of liquid films to let gas through), but the researchers discovered that two other key factors come into play. On one hand, the adhesion between bubbles, which influences their local structure, proves important when the liquid fraction is not too high. On the other hand, gas diffusion out of the films, via capillary bridges or "Plateau borders" (which always meet at the intersection of three films and constitute most of the liquid "skeleton" of the foam), turns out to be decisive in cases where the permeability of very thin interfaces is particularly low.
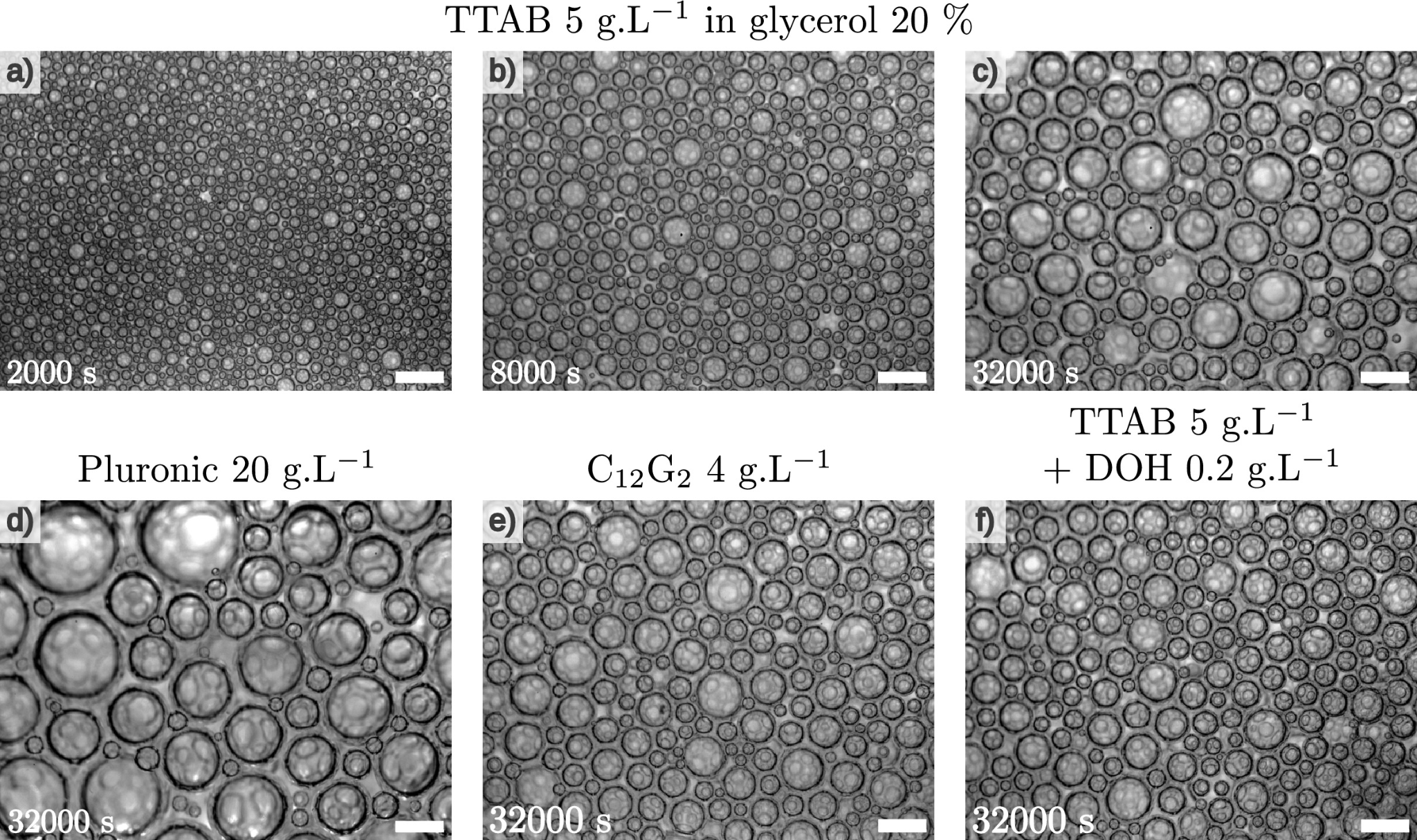
Evolution of bubble size in a wet foam observed aboard the International Space Station. The bubbles grow over time, according to a law depending on the surfactant used.
These effects can lead to either acceleration or slowing of foam coarsening, which illustrates the complexity of the general phenomenon, a complexity that this work finally makes it possible to rationalize by simultaneously taking into account the interfacial properties and structural characteristics of the studied foams.
In conclusion, this work provides a better understanding of the role of surfactant chemistry in the coarsening of wet foams. It opens perspectives for designing foams in various fields, from cosmetics to pollution control, including innovative materials. These results are published in the journal Journal of Colloid and Interface Science.