🧬 Your hair is turning gray? It's protecting you from cancer!
Follow us on Google News (click on ☆)

Pixabay Image
When the DNA of these stem cells undergoes double-strand breaks, a particularly serious type of damage, they activate a protective program called differentiation coupled with senescence. This mechanism, controlled by the p53-p21 molecular pathway, forces the stem cells to permanently mature into adult pigment cells before being eliminated from the body. This progressive loss of pigment stem cells explains why hair turns gray with age or after radiation exposure.
Under certain conditions, particularly when exposed to carcinogens like DMBA or UVB, this protective program is bypassed. The damaged stem cells then continue to divide and accumulate, supported by signals from their immediate environment. The KIT ligand molecule, secreted locally, prevents protective differentiation and maintains the cells in a state of constant renewal, thus creating favorable ground for the development of melanomas.
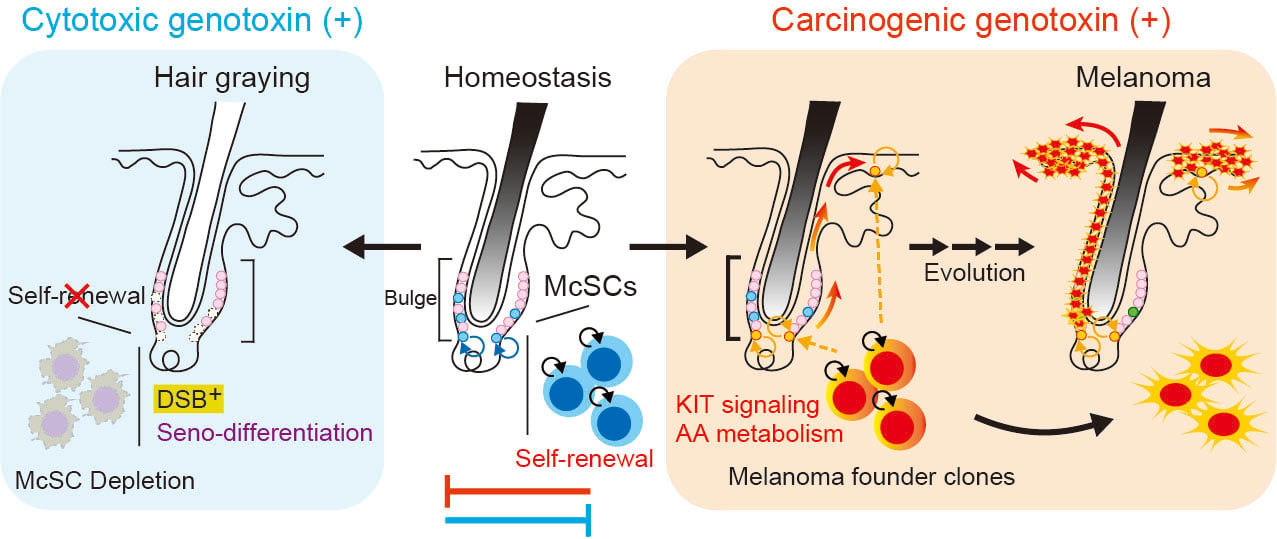
Genotoxic stresses cause divergent outcomes in melanocyte stem cells. Under the effect of cytotoxic genotoxins like X-rays, McSC renewal is impaired, leading to their depletion and graying. Carcinogenic genotoxins instead activate KIT signaling and modify arachidonic acid metabolism, promoting the appearance of precancerous clones.
Credit: Emi K. Nishimura from the University of Tokyo, Japan
This discovery establishes a fundamental link between tissue aging and carcinogenesis. The natural elimination of potentially dangerous stem cells, although it leads to hair whitening, actually represents a protective mechanism against cancer. Understanding these molecular pathways opens perspectives for modulating stem cell responses to environmental aggressions.
Cellular senescence: a natural brake on cancer
Senescence is a state in which cells permanently stop dividing while remaining metabolically active. This phenomenon constitutes an important barrier against cancerous transformation, as it prevents the proliferation of cells with damaged DNA.
Senescent cells emit various signaling molecules that alert the immune system and promote their own elimination. This cleaning process, called senolysis, maintains tissue integrity by removing potentially dangerous elements.
With age, the accumulation of senescent cells in different organs contributes to physiological aging. However, in the case of pigment stem cells, their entry into senescence coupled with differentiation represents a beneficial sacrifice for overall health.
Current research explores how to modulate senescence to prevent both premature aging and cancer development, seeking a balance between protective elimination and functional preservation.