The origin of "the molecule that made the Universe" 🌟
Follow us on Google News (click on ☆)

In a study published in Nature Communications, scientists from Michigan State University explored how H₃⁺ forms in specific organic compounds. They identified a mechanism of "molecular roaming" where, after double ionization, a dihydrogen molecule moves to capture an additional proton, thus forming H₃⁺.
This discovery expands our understanding of the formation of H₃⁺, a crucial molecule for interstellar chemistry and star formation. The researchers used a combination of ultrafast laser spectroscopy and computational chemistry to observe this phenomenon.
The molecular roaming mechanism represents a significant advance over the traditional "Coulomb explosion" theory. It shows that, in some cases, ionized molecules do not immediately separate but interact in complex ways to form H₃⁺.
The implications of this research are vast. By identifying new sources of H₃⁺, scientists can better understand chemical processes in space, including the formation of stars and complex organic molecules.
The researchers have also developed predictive factors to determine which organic compounds can produce H₃⁺ through this mechanism. These tools are valuable for future studies on cosmic chemistry.
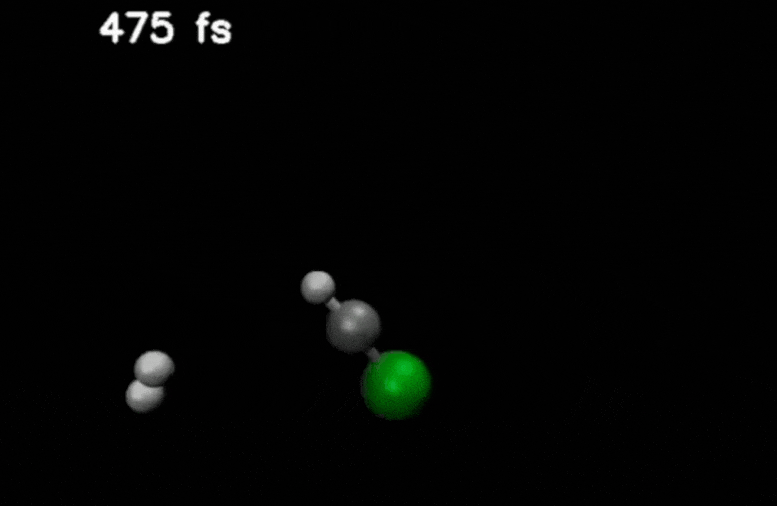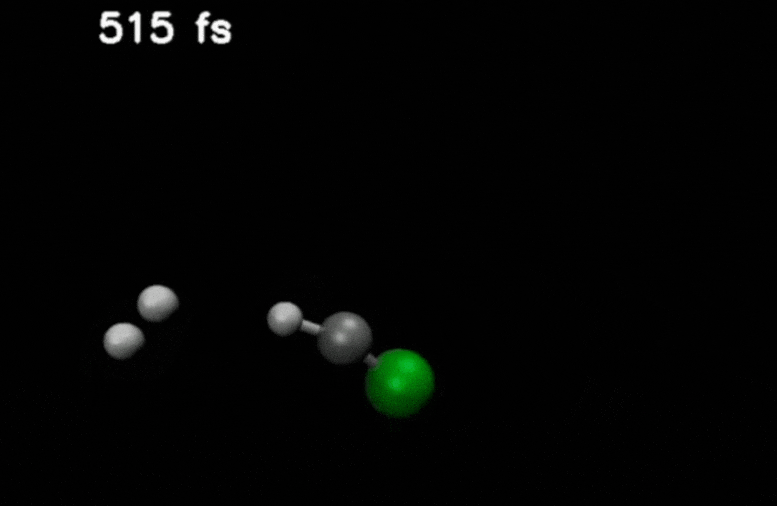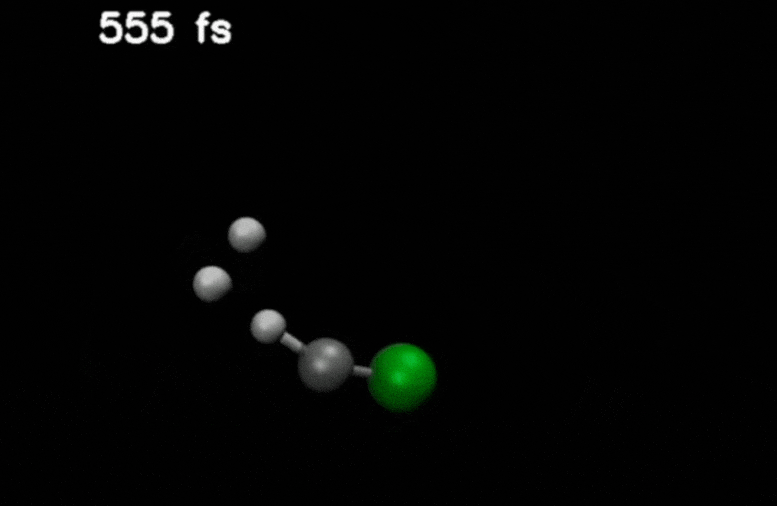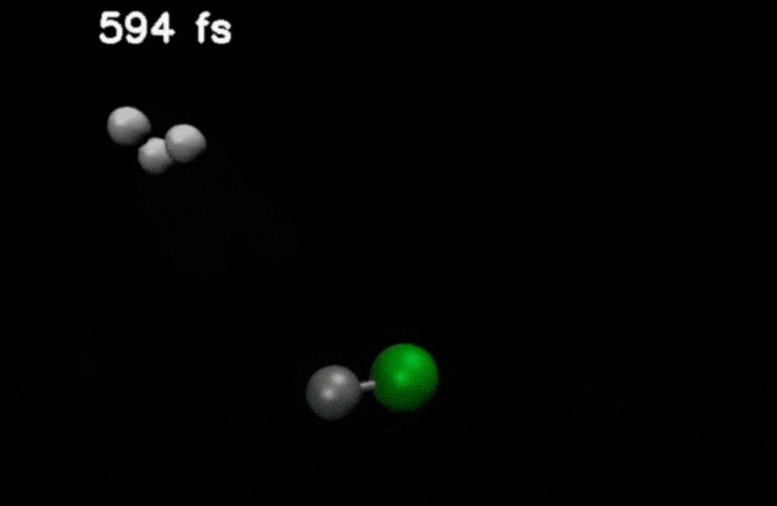
After double ionization in compounds such as methyl chloride, an H₂ molecule is ejected and moves through the molecule. It eventually captures an additional hydrogen to form H₃⁺.
Credit: Stamm, J., Priyadarsini, S.S., Sandhu, S. et al.
Finally, this study highlights the importance of H₃⁺ in the Universe. Although this molecule is less well-known than water or proteins, its role in interstellar chemistry is fundamental. The findings of this research may require a revision of current models of star formation.
What is the molecular roaming mechanism?
The molecular roaming mechanism is a process where a dihydrogen molecule, after being ejected from an ionized compound, moves around the parent molecule. Instead of immediately moving away, it interacts with other atoms to form a new molecule, such as H₃⁺.
This phenomenon is observed under specific conditions, particularly after double ionization, where a molecule loses two electrons. The roaming mechanism contrasts with the Coulomb explosion, where positive charges repel atoms, causing rapid separation.
The discovery of this mechanism has allowed scientists to better understand how H₃⁺ can form in various cosmic environments. This opens new perspectives for the study of interstellar chemistry and star formation.
Why is H₃⁺ crucial for cosmic chemistry?
H₃⁺, or trihydrogen, is often referred to as "the molecule that made the Universe" due to its central role in interstellar chemistry. It is essential for the formation of stars and complex organic molecules in space.
This molecule acts as a catalyst in many interstellar chemical reactions. It facilitates the formation of more complex molecules by interacting with other atoms and molecules in molecular clouds.
The presence of H₃⁺ in gas giant planets like Jupiter and Saturn also demonstrates its importance in various cosmic environments. Understanding its sources and behavior is therefore crucial for deciphering the chemical processes of the Universe.