Follow us on Google News (click on ☆)
By analyzing the isotopic composition of iron (δ⁵⁶Fe), scientists from LEGOS, in collaboration with American institutions, have been able to highlight a key atmospheric transformation process. These results shed new light on the mechanisms of transfer and transformation of matter from continents to oceans.

Example of a phytoplankton bloom, photosynthetic organisms, in the South Atlantic Ocean off the coast of Argentina.
Image NASA
Photosynthetic organisms need iron to perform essential functions such as respiration, photosynthesis, or nutrient assimilation. In half of the ocean, the very low concentrations of iron (less than one gram per 100 million liters of water) limit this primary production: algae can be described as "anemic."
By thus controlling the growth of phytoplankton, the structure of ecosystems, and the intensity of primary production, iron ultimately influences the carbon cycle. It is therefore a key element in the study of ocean biogeochemical cycles and climate.
A fine isotopic analysis to track aerosols
The Eastern Equatorial Pacific is one of these regions where iron is limiting. It is brought there by ocean currents that transport iron of sedimentary origin and by atmospheric dust. The latter can be of natural origin (rock, soil, etc.) or anthropogenic (ash from various sources, exhaust fumes, etc.).
A team of researchers from LEGOS and the University of Washington adopted a low-carbon strategy by using existing data from the EUCFe (Equatorial Undercurrent Fe) campaign conducted aboard the oceanographic vessel Kilo Moana between August and October 2006. The concentrations and isotopic compositions of iron from aerosols collected over the equatorial and tropical Pacific Ocean were analyzed. These areas were previously undocumented.
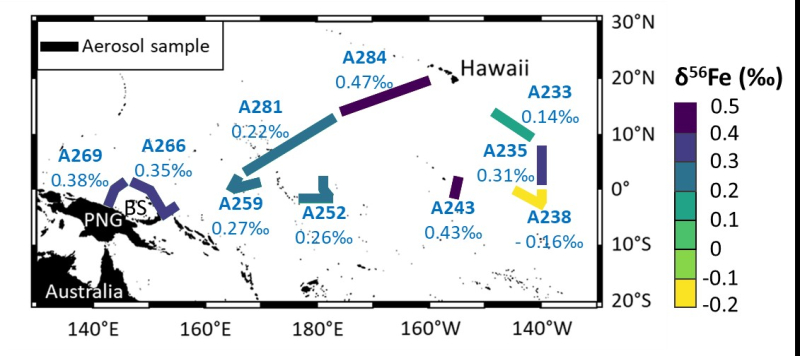
Location of aerosol samples. Aerosol sampling transects are shown by the thick lines. The Fe isotopic compositions are indicated by the color bar and under the sample names. PNG stands for Papua New Guinea. BS stands for Bismarck Sea.
First in situ evidence of atmospheric transformations
The enrichments in heavy isotopes observed in these aerosols suggest that a significant proportion of the iron in aerosols (~13%) is dissolved and then separated from the solid phase during atmospheric transport. This process, previously observed only in the laboratory, is documented here for the first time in the natural environment.
These observations highlight the importance of iron isotopes as tracers of atmospheric transformations of iron, thereby improving our understanding of the inputs of bioavailable iron to the oceans.
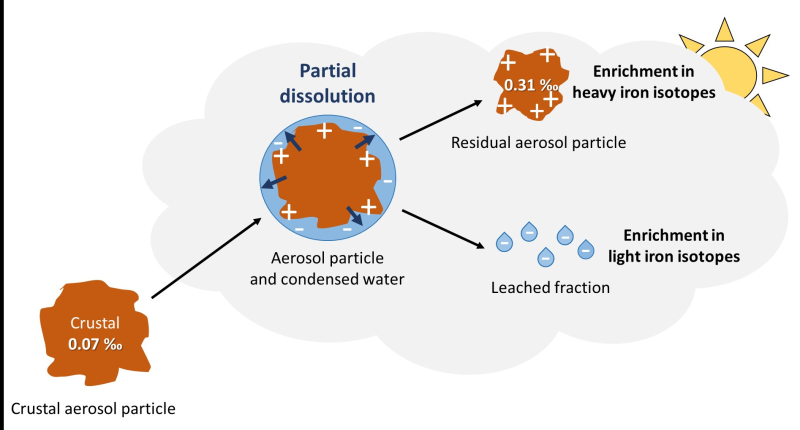
Diagram of an aerosol's journey during atmospheric transport. Partial dissolution leads to the separation of a leached fraction, enriching the residual particle in heavy iron isotopes, while the dissolved fraction becomes enriched in light isotopes.