These researchers use LED light to eliminate the most persistent pollutants
Published by Cédric,
Article author: Cédric DEPOND
Source: Angewandte Chemie International Edition
Other Languages: FR, DE, ES, PT
Article author: Cédric DEPOND
Source: Angewandte Chemie International Edition
Other Languages: FR, DE, ES, PT
Follow us on Google News (click on ☆)
PFAS, often referred to as "forever pollutants" due to their extreme persistence, are ubiquitous in the environment. They are found in common products such as food packaging, textiles, and firefighting foams.
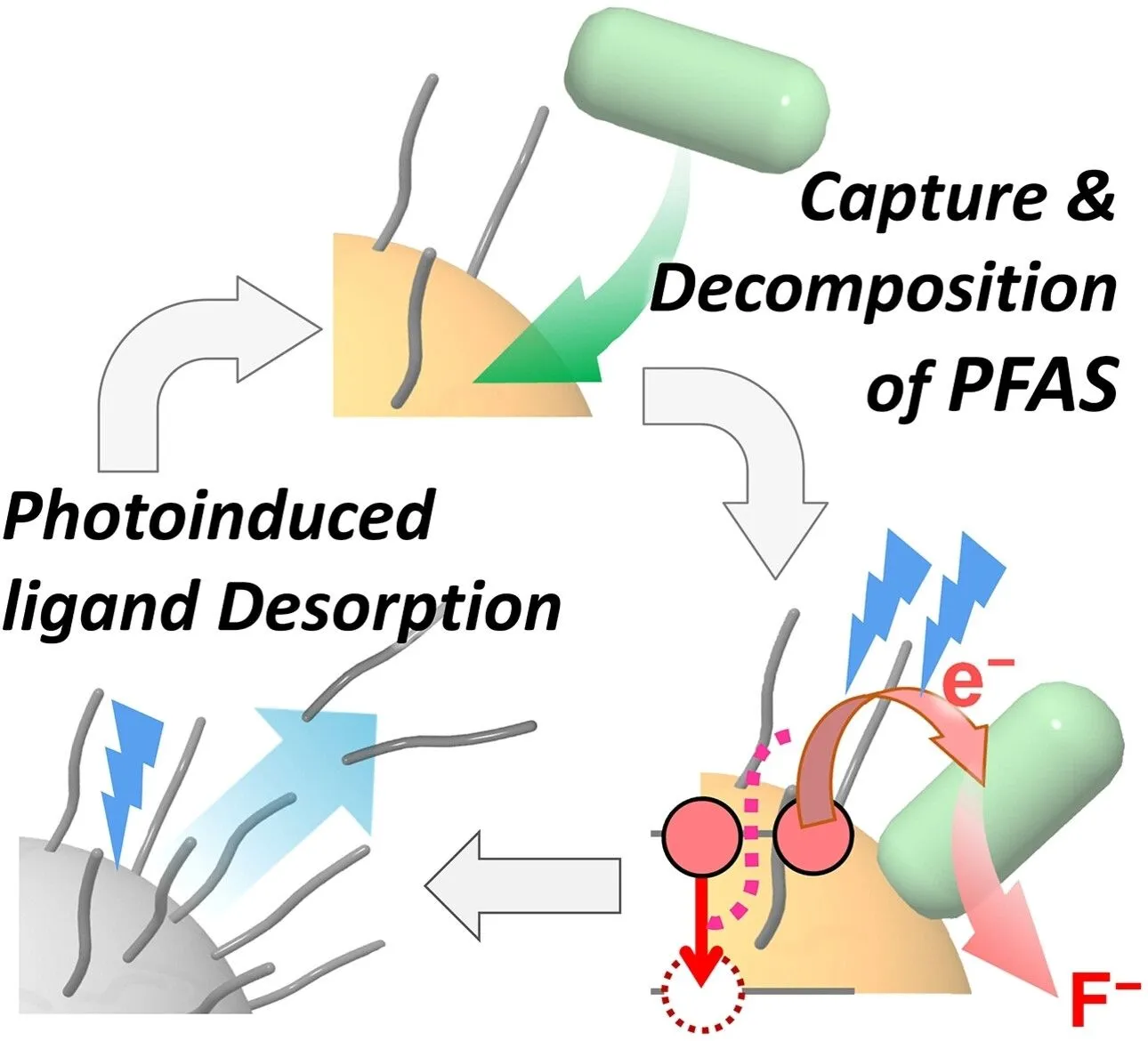
Perfluoroalkyl substances and fluorinated polymers have been effectively decomposed into fluoride ions under ambient conditions through visible LED light irradiation on semiconductor nanocrystals. This decomposition is due to cooperative mechanisms involving light-induced ligand displacement and Auger effect-mediated electron injections via hydrated electrons and higher excited states.
Their resistance to traditional decontamination processes, which generally require very high temperatures, makes their removal complex and costly. However, a team from Ritsumeikan University in Japan has developed a new approach using LED light and semiconductor nanocrystals to effectively break down these pollutants at room temperature.
This technique relies on a photocatalytic reaction, triggered by LEDs emitting at a wavelength of 405 nm (nanometers). The researchers used copper-modified cadmium sulfide (CdS) nanocrystals combined with water and triethanolamine (TEOA).
When a PFAS compound, such as perfluorooctanesulfonate (PFOS), is placed in this solution and exposed to LED light, the excited nanocrystals attract the PFOS molecules and break their carbon-fluorine bonds. This reaction allows for the complete removal of fluorine, a process which, according to the researchers, takes about eight hours.
The major advantage of this technique lies in its efficiency at much lower temperatures than traditional methods, around 100°F (38°C) compared to the usual approximately 750°F (400°C). Furthermore, it offers the potential to recycle the recovered fluorine, a crucial element in various industries, such as clean energy and pharmaceuticals.
This innovation opens up new avenues for more sustainable management of PFAS, which continue to pose significant environmental challenges. If perfected and adopted on a large scale, this method could significantly contribute to reducing pollution associated with PFAS.