This common mineral could have sparked life, here's how 🧬
Follow us on Google News (click on ☆)
Alumina, or α-alumina, is a mineral abundant in Earth's crust. According to a study published in Science Advances, its surfaces may have served as a platform for assembling amino acids, the building blocks of life. This discovery opens new perspectives on the chemical origins of life.
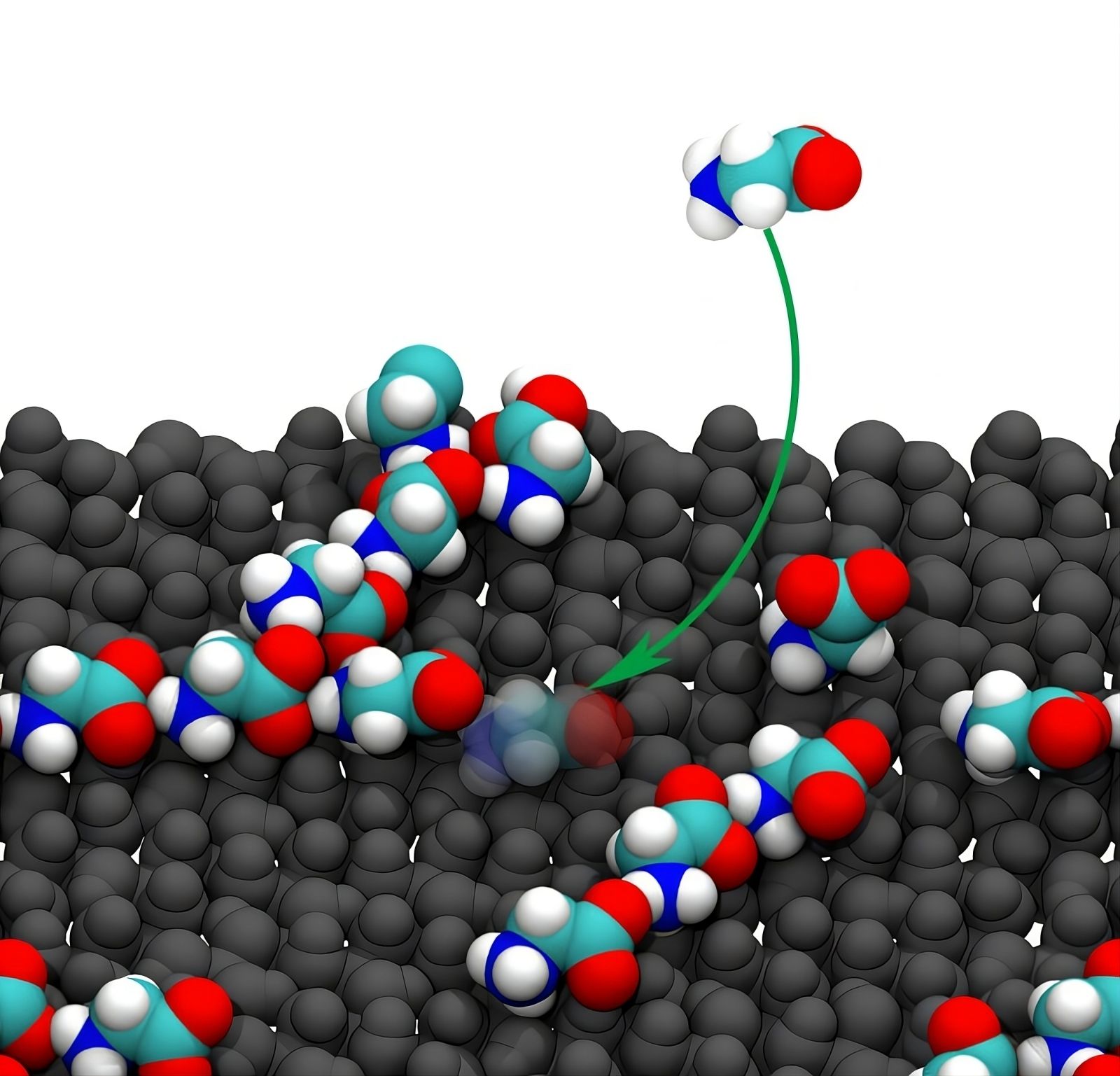
Adsorption of glycine on an alumina surface.
Credit: Ruiyu Wang.
Molecular dynamics simulations have shown that alumina acts as a microscopic template. It attracts and organizes glycine molecules, significantly increasing their likelihood of forming chains. This process is up to 100,000 times more efficient than in water alone.
The interface between the mineral and water creates a zone where amino acid concentration is high. This local density promotes the chemical reactions needed for polymerization. The molecules align according to the atomic structure of alumina, stabilizing the formed chains.
Water, often considered a simple solvent, plays a crucial role here. Water molecules surrounding amino acids must be displaced to allow their assembly. Alumina facilitates this process by optimally orienting the glycine molecules.
These results shed light on the mechanisms that may have led to the emergence of life. They suggest that minerals could have provided a favorable environment for the formation of the first biomolecules. This hypothesis strengthens the idea of a mineral origin of life.
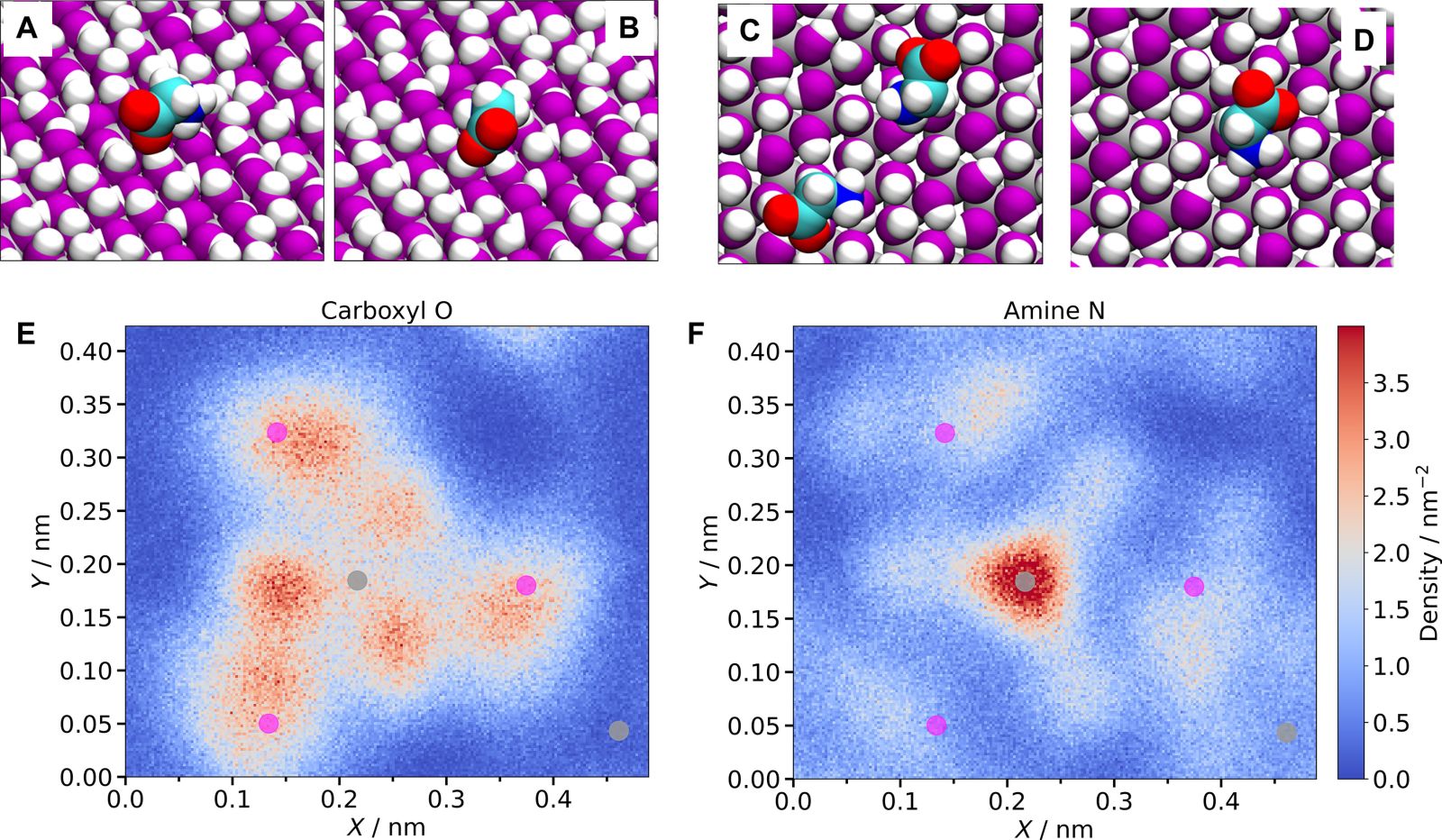
a) Glycine accepts a hydrogen bond with an AlOH group via its carboxyl group.
b) Glycine accepts two hydrogen bonds via its carboxyl group.
c) Glycine donates one (bottom) or two (top) hydrogen bonds via its amine group.
d) Glycine donates three hydrogen bonds via its amine group.
e) 2D distribution of carbon atoms from the carboxyl group on the α-alumina (0001) surface.
f) 2D distribution of nitrogen atoms from the amine group on the same surface. Purple and gray dots indicate oxygen and aluminum atoms on the surface.
The potential applications of these discoveries are vast. By drawing inspiration from these natural processes, scientists could develop new biomimetic materials. These innovations could revolutionize fields like medicine or biotechnology.
This study highlights the importance of interactions between minerals and organic molecules. It provides a serious lead for understanding how life could have emerged from inert matter. Research continues to explore other minerals and their possible roles in the origin of life.
Focus: how can minerals catalyze the formation of complex molecules?
Minerals like alumina have surfaces with unique properties. These surfaces can adsorb organic molecules, align them, and concentrate them. This spatial organization promotes chemical reactions between the molecules.
Adsorption also reduces the energy required for reactions to occur. Minerals thus act as natural catalysts. They accelerate processes that would otherwise be too slow or unlikely under normal conditions.
Moreover, the crystalline structure of minerals imposes order on adsorbed molecules. This order can lead to the formation of more complex and stable structures. It is a key mechanism for understanding how the first biomolecules could have formed.
What role does water play in amino acid assembly?
Water is essential for life, but it can also be an obstacle. Water molecules form a layer around amino acids, sometimes preventing them from coming close. For these amino acids to react, this water layer must be displaced.
Mineral surfaces can facilitate this process. By adsorbing amino acids, they partially free them from their water envelope. This allows the molecules to come close enough to react with each other.
Water also influences the structure of adsorbed molecules. Depending on its presence or absence, amino acids can adopt different conformations. This flexibility is crucial for the formation of complex polymeric chains.