This innovation efficiently synthesizes fuel with water and the Sun ⛽
Follow us on Google News (click on ☆)
The idea relies on a natural but complex process: splitting water molecules into oxygen and hydrogen using photocatalysts. These photocatalysts, when exposed to light, initiate chemical reactions that release hydrogen, a key energy resource. However, existing systems struggle to achieve sufficient efficiency for large-scale use.

Aerial view of a 1,076 sq. ft. (100 m²) operational photocatalyst system for solar hydrogen production. The system uses 1,600 panel reactor units (each 96.88 sq. in. [625 cm²]), where 48 panel reactor units are integrated to form a 32.3 sq. ft. (3 m²) module, and 33 and one-third (33 + 1/3) modules are connected to create the system. The panel reactor is linked to a gas separation facility.
In detail, the developed prototype relies on photocatalytic sheets capable of capturing solar energy to decompose water molecules. Unlike conventional systems, this reactor uses a two-step configuration, known as the Z-scheme, which effectively separates oxygen and hydrogen.
This approach not only improves the efficiency of the process but also reduces the risks associated with the formation of explosive gases like oxyhydrogen. Through this method, researchers demonstrated near-perfect energy conversion under ultraviolet light, an important milestone for optimizing photocatalysts.
The team designed a 1,076 sq. ft. (100 m²) reactor capable of operating outdoors with natural light, proving the feasibility of large-scale deployment. This device incorporates advanced safety measures to manage potentially hazardous by-products while allowing the direct separation of hydrogen and oxygen. This configuration promises a safer and more sustainable solution for hydrogen production.
Furthermore, developing photocatalysts sensitive to visible light remains a priority, as it would allow for the capture of a larger portion of the solar spectrum and further improve overall efficiency.
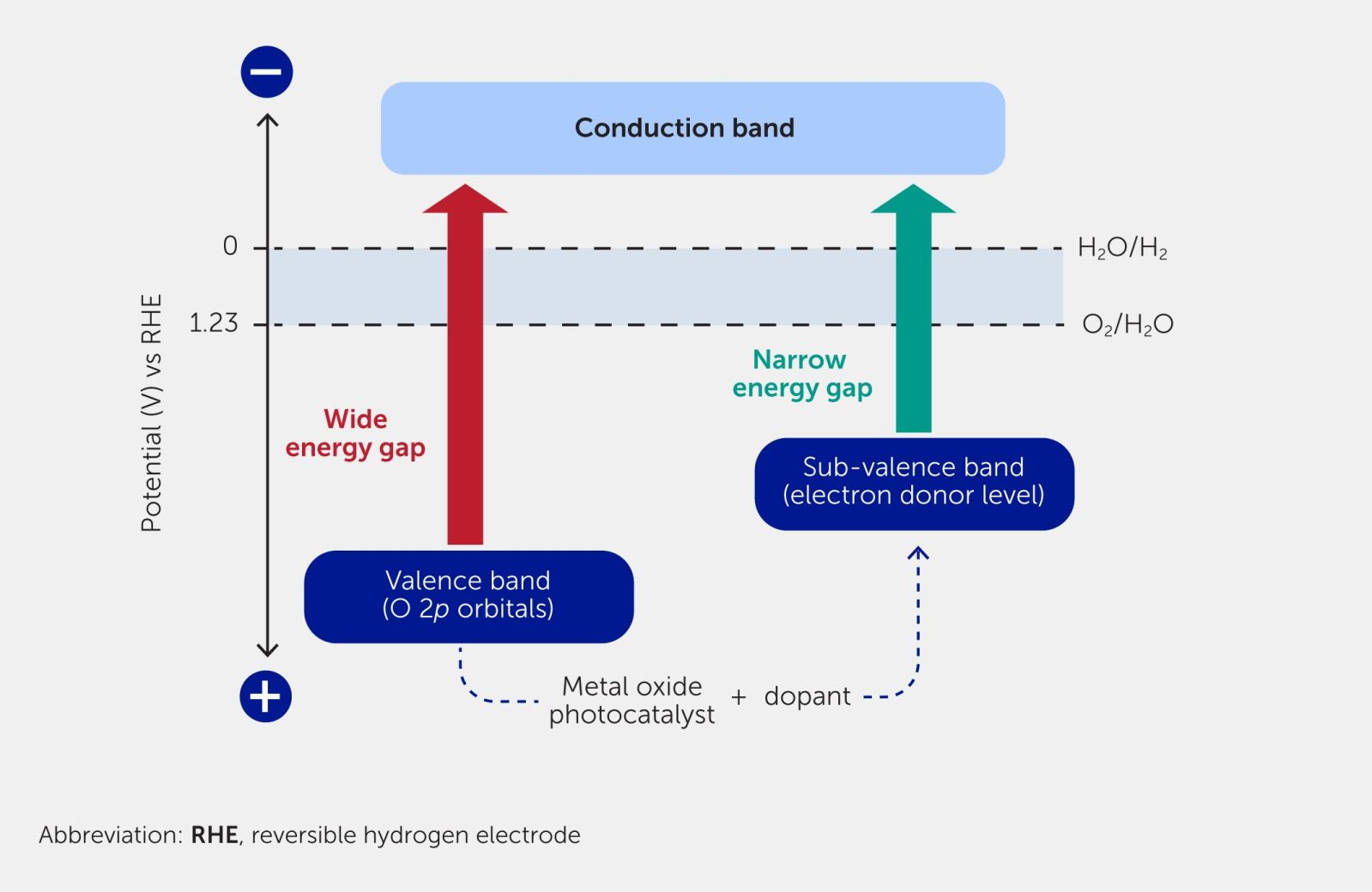
The sensitization of a wide-band photocatalyst is achieved by doping with transition metals. The doping elements create impurity energy levels, reducing the energy required for photoexcitation and rendering the material reactive to visible light.
According to Professor Kazunari Domen, this advancement could transform the conversion of solar energy into storable chemical energy, provided the catalysts are improved. Currently, their efficiency barely reaches 5% under real-world conditions.
The researchers also emphasize the role of public policies. A notable breakthrough in this technology would encourage governments and companies to invest in such infrastructure and revise regulations concerning solar fuels.
Beyond the technical aspect, the ecological potential is immense. A clean and renewable fuel accessible on a large scale could significantly reduce our dependence on fossil fuels, marking a crucial step toward a sustainable energy transition. This discovery demonstrates that the road to commercial adoption is still long. However, the first steps are promising, and the commitment of scientists could pave the way toward a greener energy future.
What is photocatalysis, and why is it key for hydrogen?
Photocatalysis is a chemical reaction accelerated by a catalyst activated by light, often sunlight. It is essential for breaking down molecules like water into its base elements: hydrogen and oxygen.
Photocatalysts absorb light energy, exciting their electrons to initiate the reaction. These energized electrons then participate in breaking the chemical bonds of water.
In the quest for clean fuel, this process enables the production of hydrogen without CO₂ emissions. However, its theoretical simplicity masks practical challenges: it requires high-performance materials and efficient systems.