This liquid uranium defies known laws of physics
Published by Cédric,
Article author: Cédric DEPOND
Source: Journal of the American Chemical Society
Other Languages: FR, DE, ES, PT
Article author: Cédric DEPOND
Source: Journal of the American Chemical Society
Other Languages: FR, DE, ES, PT
Follow us on Google News (click on ☆)
At Oak Ridge National Laboratory (ORNL), a team analyzed this unexpected behavior. Using a combination of modeling and experiments with the Spallation Neutron Source (SNS), they uncovered properties never before observed.
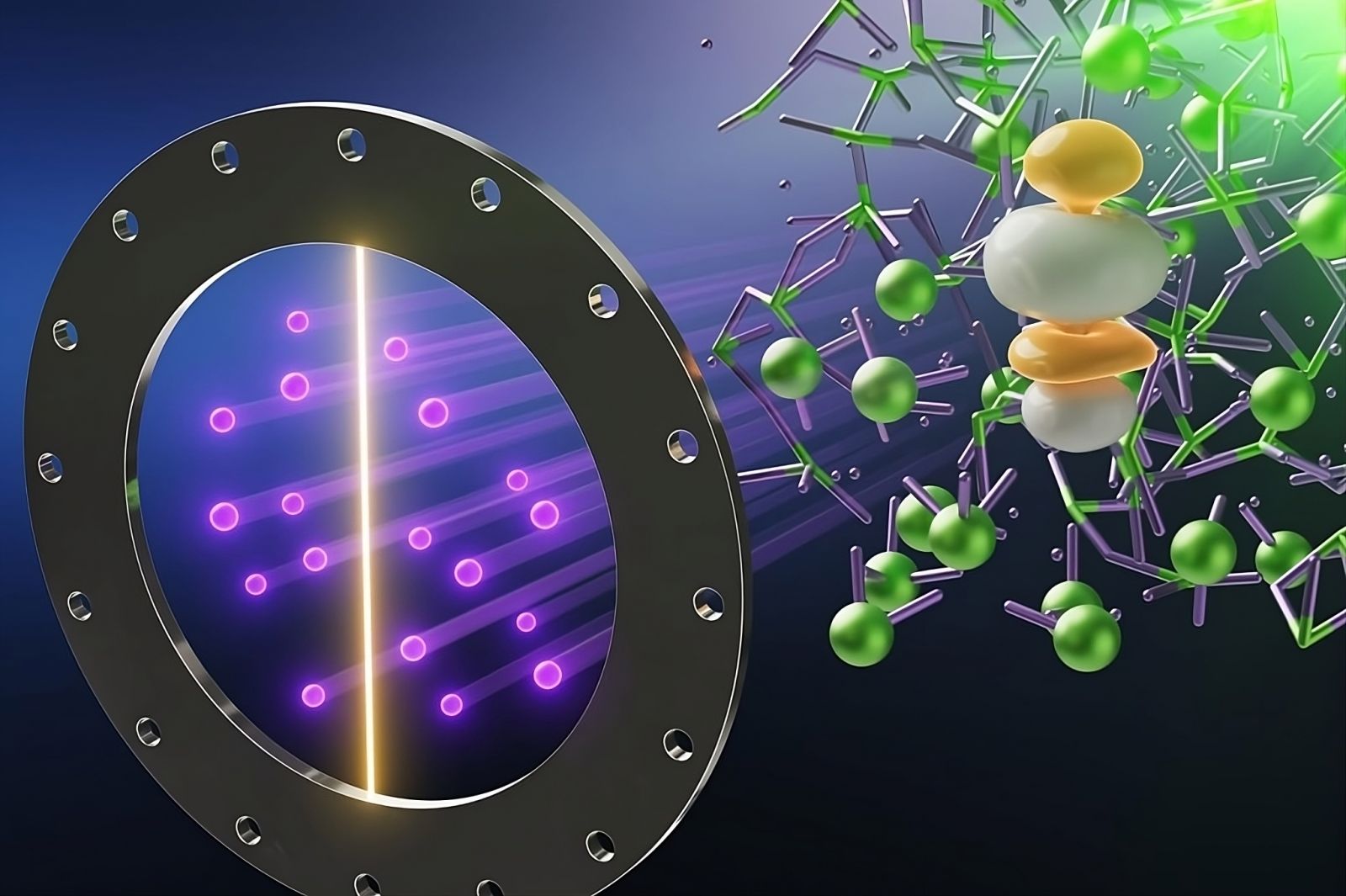
In this illustration, neutrons produced at the SNS (purple dots) scatter from molten UCl3, shown in green, revealing its atomic structure. The yellow and white globules (simulated data) represent the oscillating bonds of UCl₃.
Credit: Alex Ivanov/ORNL, U.S. Department of Energy
For the first time, scientists observed how uranium trichloride (UCl₃) - or uranium salt - contracts when heated. A surprising property, as most materials tend to expand under heat.
This breakthrough was made possible thanks to the ultra-efficient instruments of the SNS, one of the brightest sources of neutrons in the world. By bombarding the molten uranium salt with neutrons and analyzing their scattering patterns, they could precisely map its atomic structure.
When examining the chemical bonds of UCl₃ at the atomic scale, researchers observed bond lengths that contract and expand cyclically at an extremely fast rate. This movement reveals a switch between covalent and ionic states, which is highly unusual for this type of salt.
These results shed new light on the behavior of actinides, such as uranium, at extreme temperatures. This discovery could lead to the development of safer and more efficient nuclear reactors, while also opening new avenues for handling nuclear waste.
The research was conducted as part of the U.S. Department of Energy's "Molten Salts in Extreme Environments" initiative. This project involves collaborations with renowned laboratories such as Brookhaven and Argonne.
These new findings challenge existing theories and open up unexpected possibilities for the nuclear energy of tomorrow.
Uranium trichloride (UCl₃) in detail
Uranium trichloride (UCl₃) is a uranium salt where uranium is bonded to three chlorine atoms. This compound plays a key role in scientific research, particularly in studying the chemical and physical properties of uranium-containing materials. UCl₃ is frequently used in specialized laboratories to explore complex chemical reactions or to synthesize other uranium-based compounds.
Like all uranium salts, uranium trichloride is handled with great care. Its radioactivity and toxicity require strictly controlled working conditions to protect researchers and prevent contamination. Laboratories working with this compound are equipped to mitigate exposure risks, using specialized protective equipment and rigorous safety protocols.
Outside of scientific research, UCl₃ has few direct industrial applications due to the risks involved in handling it. Nevertheless, it remains an essential component in certain areas of nuclear physics and chemistry, where it is used to deepen our understanding of radioactive elements and their interactions.