Follow us on Google News (click on ☆)
Among these diseases is the infamous sleeping sickness, caused by a parasite called Trypanosoma brucei. This parasite is transmitted to humans and animals through the bite of the tsetse fly. In humans, it causes severe neurological disorders, and in animals, it leads to nagana, a disease that heavily affects livestock.
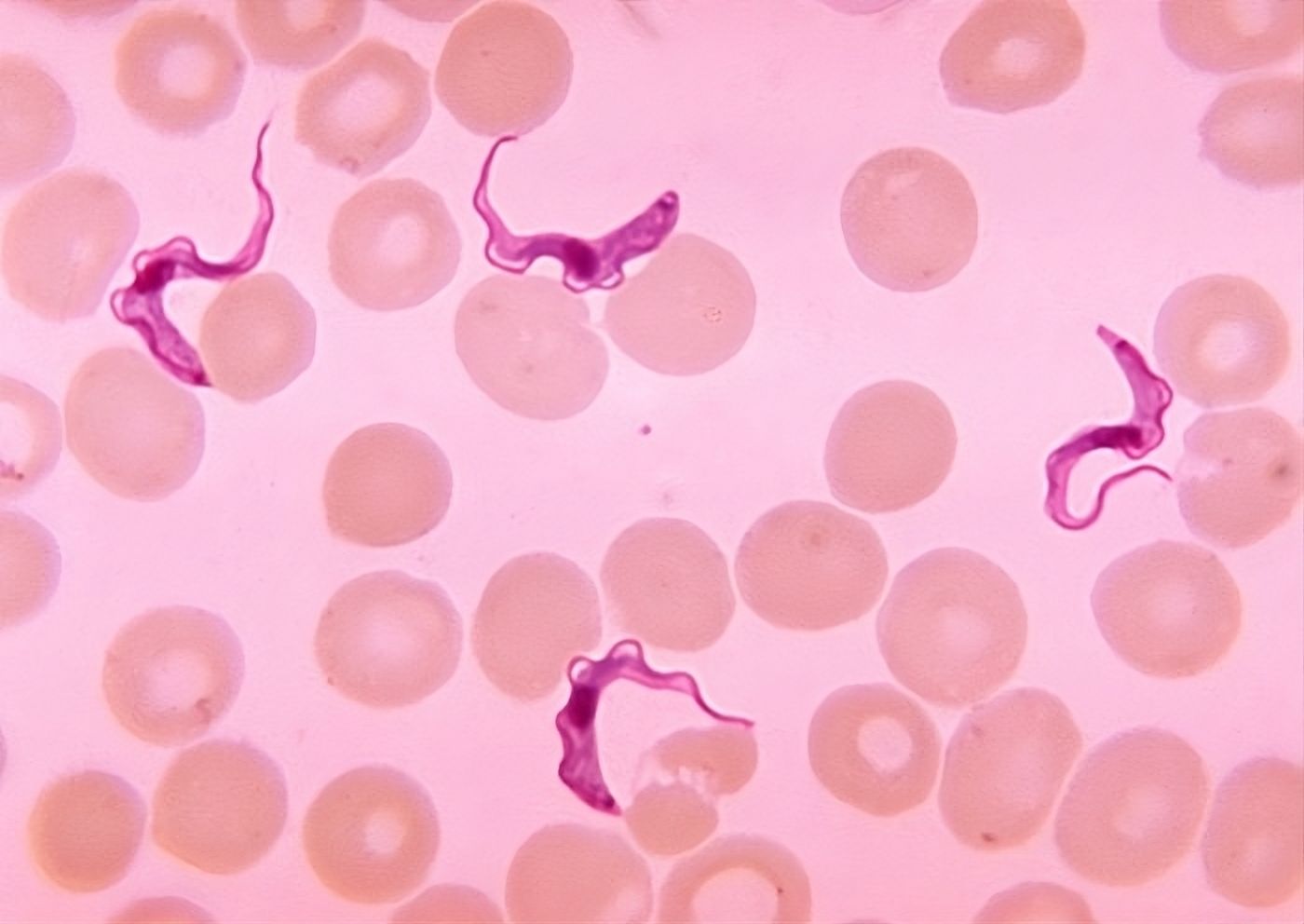
Trypanosoma brucei in the blood of a patient suffering from African human trypanosomiasis, commonly known as sleeping sickness.
Image Wikimedia
The situation worsens when considering that current treatments are scarce, toxic, and increasingly ineffective due to the emergence of resistance.
In this parasite, a unique mitochondrion changes shape and function
Unlike most living cells, which have multiple mitochondria (the cell's "powerhouses"), Trypanosoma brucei has only one, which completely changes shape and function depending on the environment, involving membrane fusion and fission mechanisms.
In the tsetse fly, the parasite's mitochondrion takes on a reticulated form, large and highly active, producing energy through a complex process called oxidative phosphorylation. But once in the mammal's bloodstream, the parasite's mitochondrion shrinks significantly into a simple tube shape, and the parasite mainly uses sugars (glucose) to produce energy through a much simpler process: glycolysis.
In most living organisms, mitochondria are highly dynamic: they constantly fuse or divide to adapt to the cell's needs. These processes, well-documented in mammalian cells or in the yeast Saccharomyces cerevisiae (baker's yeast), are regulated by proteins from the dynamin family. These proteins have a GTP-hydrolyzing domain and structures that allow them to bind to membranes and oligomerize.
In Trypanosoma brucei, the mitochondrion forms a unique and continuous network, highly peculiar, which only divides when the cell itself divides. Until recently, only proteins involved in fission (mitochondrial division) had been identified in this parasite. The proteins responsible for fusion, present in humans or yeast (such as Mfn or Opa1), seemed simply absent, suggesting a different fusion mechanism.
A new dynamin-family protein could explain this peculiarity
In a paper published in the journal Current Biology, scientists discovered a new type of dynamin-family protein in Trypanosoma brucei, named TbMfnL (Trypanosoma brucei Mitofusin-Like). This protein appears to originate from an ancient protein family, present in many living organisms (including some bacteria), but absent in mammals and yeast. It could therefore be an ancient remnant of a cellular membrane remodeling mechanism.
Their results show that TbMfnL is anchored in the inner mitochondrial membrane, on the matrix side (the interior of the mitochondrion), which is unprecedented for proteins remodeling mitochondrial membranes. By increasing the production of TbMfnL in the cell, scientists observed a significant increase in the interconnection and branching of mitochondrial filaments, a mechanism dependent on GTP hydrolysis, which provides the necessary energy. In other words, TbMfnL could modulate the shape of the mitochondrion from the inside, via a mechanism entirely different from that observed in other eukaryotes.
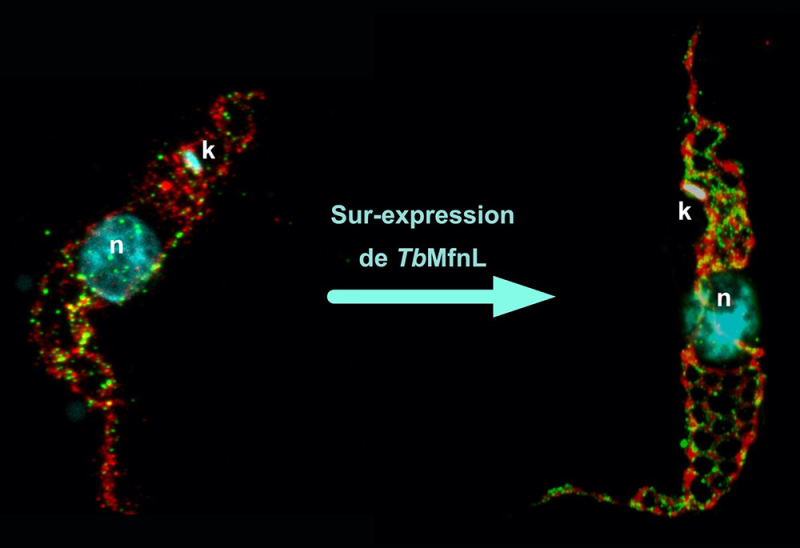
Labeling of the mitochondrion of a wild-type trypanosome and one overexpressing the TbMfnL protein using expansion microscopy. Red, labeling of the mitochondrial matrix (Threonine dehydrogenase, Tdh); Green, membrane labeling of the TbMfnL protein; Cyan, labeling of the nucleus (n) and mitochondrial DNA (k, kinetoplast).
© Emmanuel Tetaud
This discovery paves the way for a new understanding of mitochondrial membrane structuring mechanisms and, more broadly, cellular membranes, not only in Trypanosoma brucei but also in other organisms. In the long term, this discovery could enable more effective targeting of the parasite by attacking an essential and unique element. And perhaps, develop more specific, less toxic treatments that spare human cells.