Follow us on Google News (click on ☆)
An international team centered around the CNRS, the Soleil synchrotron, and universities in Lille, Nantes, Toulouse, and California has created electrodes made of ruthenium nitride (RuN) that exhibit exceptional performance. Published in Nature Materials, this research also includes methodological developments for studying the electrochemical processes in supercapacitors.
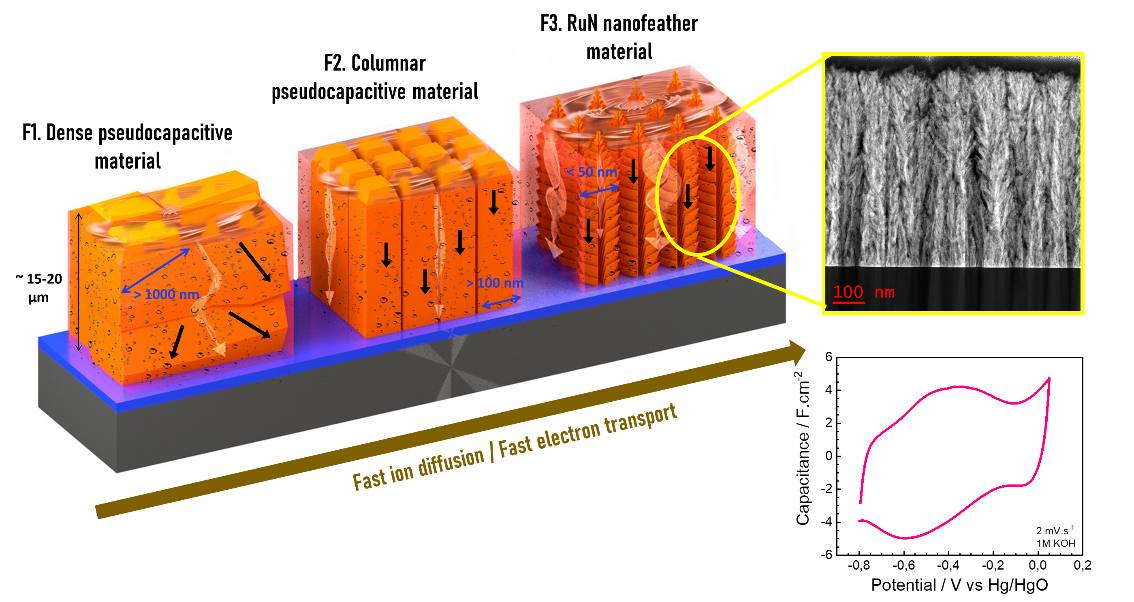
Comparison of various electrodes, arranged from left to right by the speed of ion diffusion and electron transport. The ruthenium nitride electrode is on the far right.
© Anne Duchene, Huy Dinh Khac, and Christophe Lethien
Supercapacitors store electrical energy while offering greater power and faster charging speeds than batteries, though they have less storage capacity. A large Franco-American group, including researchers from the CNRS, Soleil synchrotron, and universities in Lille, Nantes, Toulouse, and California, developed ruthenium nitride (RuN) based electrodes. These electrodes deliver a capacitance of over 500 farads per cubic centimeter (F.cm-3). This capacity increases to 3200 F.cm-3 following an electrochemical oxidation process that was analyzed in real-time (operando) using synchrotron radiation.
These electrodes showcase time constants below ten seconds, even before oxidation, making them strong candidates for rapid charging devices. By comparison, MXene-based electrodes—two-dimensional state-of-the-art materials—achieve about 1500 F.cm-3 with similar time constants.
The new electrodes rely on ruthenium nitride (RuN) films, deposited through an optimized sputtering method as part of this study. The material takes the form of arrays of porous nanofeathers, with cores of ruthenium nitride and rims of ruthenium oxide (RuO2).
During electrochemical oxidation, RuO2 hydrates to become h-RuO2 and the site of rapid redox reactions at the interface between the electrode and the electrolyte. The generated electrons are quickly transported by the RuN cores of the nanofeathers, which serve as particularly good electrical conductors.
Beyond the achieved performance, this study helps unveil the reaction mechanisms behind these results. It includes a segment on understanding charge storage (operando), using a suite of advanced characterizations such as X-ray absorption spectroscopy under synchrotron radiation, and transmission electron microscopy. This technology is currently being transferred and commercialized, with support from CNRS Innovation, within the start-up Hileores, derived from previous work by the Lille team.
References:
Nanofeather ruthenium nitride electrodes for electrochemical capacitors.
Huy Dinh Khac, Grace Whang, Antonella Iadecola, Houssine Makhlouf, Antoine Barnabé, Adrien Teurtrie, Maya Marinova, Marielle Huvé, Isabelle Roch-Jeune, Camille Douard, Thierry Brousse, Bruce Dunn, Pascal Roussel & Christophe Lethien.
Nature Materials, 2024.
https://doi.org/10.1038/s41563-024-01816-0