Follow us on Google News (click on ☆)

Gliomas account for 50% of brain cancers and are the most common type of brain tumors. Molecular alterations involved in these cancers primarily affect membrane receptors with tyrosine kinase activity, serving as "switches" for activating or inhibiting numerous functions such as cell division or migration. Specifically, an amplification and/or mutations of the epidermal growth factor receptor (EGFR) and the signaling pathways associated with it can be observed.
This leads to uncontrolled cell division and, eventually, the emergence of tumors. Although multiple targeted therapies have been developed, current treatments remain ineffective against glioblastomas, the most severe form of these brain cancers. Hence, it is critical to identify new therapeutic targets for treating these cancers.
Scientists from the CNRS, at the Molecular Biophysics Center and the Laboratory for Extreme Conditions and Materials: High Temperature and Irradiation, studied a model of these brain cancers in Drosophila to better understand the metabolic disturbances associated with them.
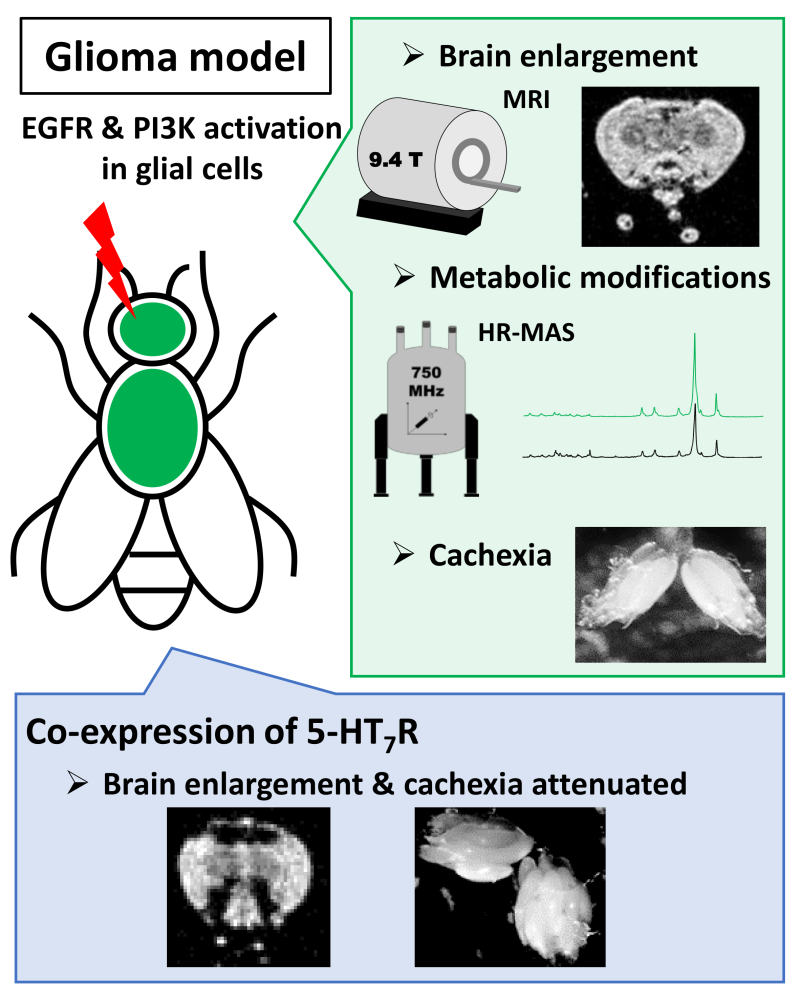
Combining MRI and NMR enables the study of biochemical mechanisms in a Drosophila brain tumor (glioma) model. A serotonin receptor, R5-HT7, emerges as an interesting therapeutic target to mitigate glioma effects.
© Séverine Morisset-Lopez
Overexpression of EGF receptors and an enzyme (phosphoinositide 3-kinase, PI3K) in glial cells—those central nervous system cells that support and protect neurons—leads to an easily MRI-detectable brain hypertrophy and the onset of cachexia (wasting of fat tissue and muscle mass (cachexia)). The researchers then investigated cellular metabolic alterations using high-resolution magic angle spinning NMR (HR-MAS) and liquid 2D NMR, identifying significant pathway modifications in the glioma, particularly those characteristic of cachexia.
While the benefits of targeting the 5-HT7 serotonin receptor for treatment of neurological and psychiatric conditions are well described, its role in controlling tumor proliferation is not as well explored. To address this question, the scientists genetically altered Drosophila carrying the glioma to express the human R5-HT7 receptor on the surface of glial cells. They demonstrated that the expression of this serotonin receptor reduces several effects associated with glioma development, such as brain hypertrophy visible through MRI, and cachexia.
The combination of NMR analysis techniques employed here, as detailed in The Faseb Journal, proves to be an effective tool for comprehending the biochemical mechanisms behind certain brain cancers, a crucial step in developing new targeted therapies.
Reference:
An adult Drosophila glioma model to highlight metabolic dysfunctions and evaluate the role of the serotonin 5-HT7 receptor as a potential therapeutic target
Marylène Bertrand, Frédéric Szeremeta, Nadège Hervouet-Coste, Vincent Sarou-Kanian, Céline Landon, Séverine Morisset-Lopez & Martine Decoville.
The Faseb Journal 2023
DOI: 10.1096/fj.202300783RR