Follow us on Google News (click on ☆)
This innovative process overcomes the limitations of other systems developed so far, which are less selective or too unstable for industrial-scale use. A breakthrough published in Nature Catalysis that paves the way for sustainable production of non-fossil fuels.
The transformation of carbon dioxide (CO₂) into higher-value chemicals, such as fuels, is a major challenge in green chemistry. Since the CO2 molecule is very stable, this transformation requires energy and the development of efficient and cost-effective catalysts.
Several electrochemical processes have been developed in recent years that use copper-based electrodes, which act as catalysts, or carbon-based electrodes coated with molecular catalysts*. So far, the latter have mainly succeeded in producing simple compounds like carbon monoxide (CO) or formate (HCOO-). Obtaining more complex products involving carbon-carbon bonds remains difficult.
While copper-based catalysts can achieve this, they often generate a mixture of molecules with two or more carbons, typically ethanol but also ethylene and acetate, with limited control over selectivity. From the perspective of a circular carbon economy, it would obviously be preferable to directly produce ethanol without needing additional purification and separation steps.
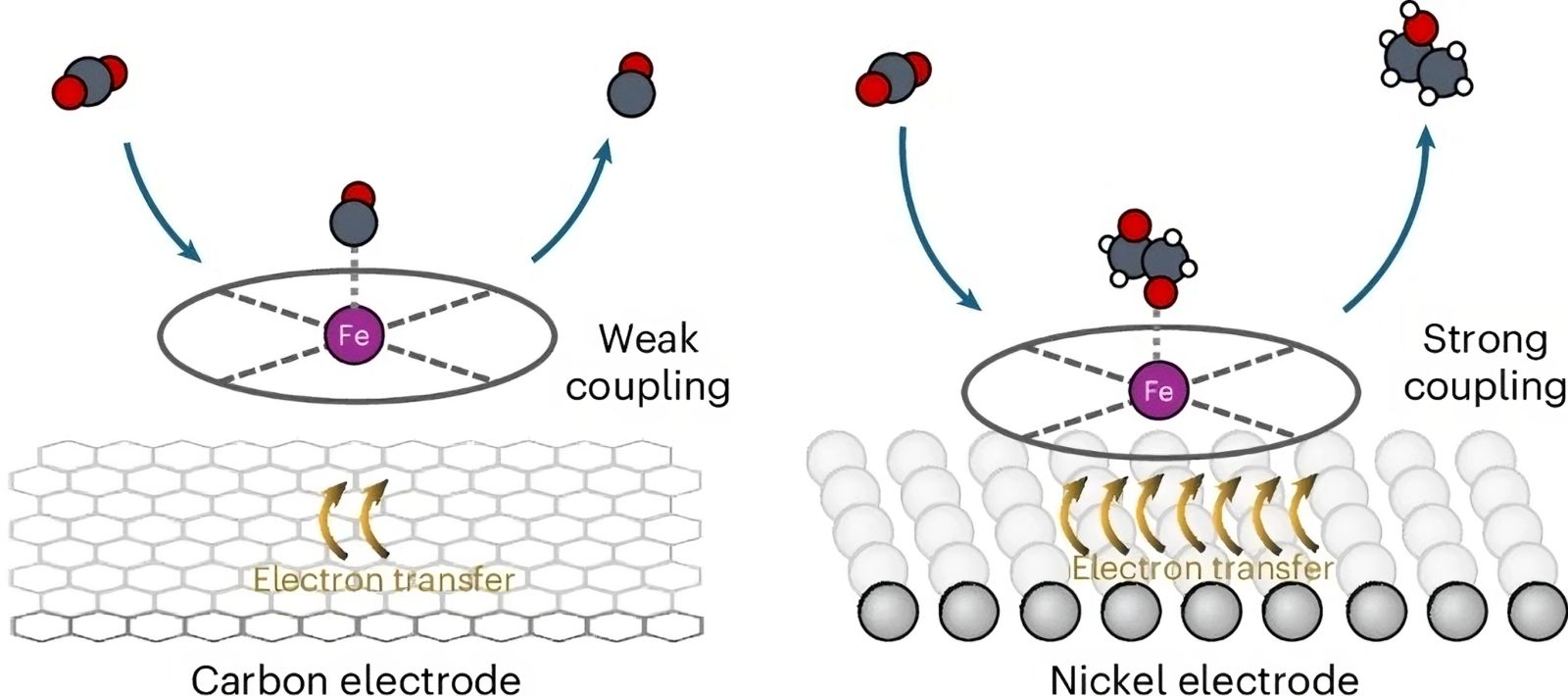
An international team of scientists has recently developed a new catalytic system that overcomes these obstacles using an organometallic iron complex, iron tetraphenylporphyrin or Fe-TPP, deposited on a nickel substrate.
The Dutch, American, Canadian, and French researchers (from the Paris Institute of Molecular Chemistry - CNRS/Sorbonne University) used an innovative approach by attaching the molecular catalyst, an iron complex, onto nickel foam. This strategy, which abandons traditional carbon supports in favor of metallic electrodes despite their potential for competing electrochemical reactions, allowed them to achieve near-complete conversion of CO2 into ethanol.
Remarkably, the nickel-Fe-TPP electrode provides high ethanol yields at low potentials without producing undesirable byproducts like acetate or methane, surpassing all non-copper-based systems. And this efficiency persists over many hours of use.
This breakthrough, published in Nature Catalysis, challenges long-standing assumptions about molecular catalysts. It also opens up new electrochemical pathways for converting CO₂ and CO into alcohols such as ethanol, but also methanol or propanol.
The ethanol produced can be easily stored and used as renewable fuel, offering a sustainable alternative to fossil resources and reducing dependence on biomass and water-intensive bioethanol production. Could the CO2 loop soon be closed?
Note:
* Unlike traditional solid or metallic catalysts, molecular catalysts are often organometallic complexes, where a central metal atom is surrounded by organic ligands that modify its chemical properties.
Writer: AVR
Reference:
Eliminating redox-mediated electron transfer mechanisms on a supported molecular catalyst enables CO2 conversion to ethanol
Maryam Abdinejad, Amirhossein Farzi, Robin Möller-Gulland, Fokko Mulder, Chengyu Liu, Junming Shao, Jasper Biemolt, Marc Robert, Ali Seifitokaldani & Thomas Burdyny.
Nature Catalysis 2024
https://doi.org/10.1038/s41929-024-01225-1