Follow us on Google News (click on ☆)

The researchers utilized a novel imaging technique, still under peer review, published on the preliminary server arXiv. They cooled lithium atoms to a temperature near absolute zero by bombarding them with photons from a laser. This process aimed to slow down their movement. Subsequently, with additional lasers, the atoms were confined within an optical lattice.
By alternating the activation and deactivation of this lattice, the atoms oscillated between a quasi-particle confined state and a wave-like state. This manipulation allowed the scientists to observe the transformation of the atoms and understand how they behave as waves over time.
According to Louis de Broglie and Erwin Schrödinger, pioneers of this concept in 1924 and 1926 respectively, all quantum-sized objects, thus all matter at a very small scale, simultaneously exist as both particles and waves. This counterintuitive property of quantum mechanics has been observed in many experiments previously but in an indirect manner.
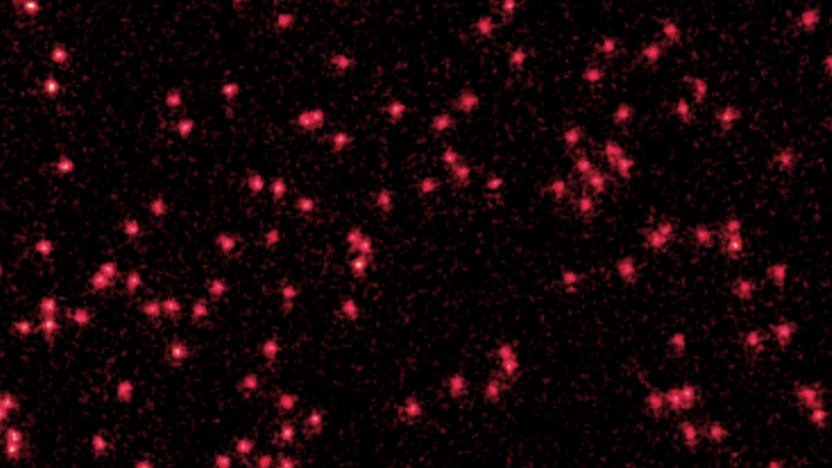
The image shows Lithium atoms cooled near absolute zero appearing as red dots.
By combining several of these images, the authors observed the atoms behaving like waves.
Credit: Verstraten et al.
The microscope used in this study captures the light emitted by the atoms, allowing for the visualization of changes in their state. This imaging technique could accelerate our understanding of complex atomic systems and shed light on some exotic states of matter, like those found in neutron star cores or the quark-gluon plasma believed to have existed just after the Big Bang.