Follow us on Google News (click on ☆)
As an alternative to fossil fuels, this fuel produced from decarbonized electricity could be used in industry or transportation. A breakthrough published in Nature Materials that could valorize CO directly emitted by certain industries and thus reduce their overall carbon footprint.

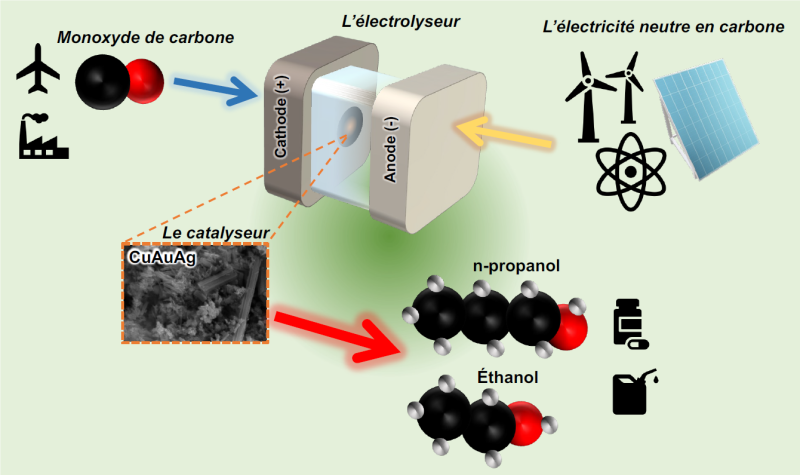
E-fuels (electrofuels) like e-ethanol are an attractive alternative to fossil fuels for use in industry and transportation, for example. Produced from decarbonized electricity, their synthesis can potentially be achieved through direct conversion of carbon dioxide (CO2), captured from concentrated emissions of metallurgical plants, fermenters, or cement factories, or carbon monoxide (CO) also emitted by certain industries.
From a chemical standpoint, these CO2 or CO electroreduction reactions are highly complex due to the large number of electrons and protons involved. They require specific electrolyzers for gas conversion and, most importantly, efficient and selective catalysts to produce only the desired and directly valorizable products.
In recent years, many efforts have focused on CO2 electroreduction as a promising pathway to produce fuels and organic molecules useful for the chemical industry. However, significant obstacles remain that prevent the scaling up of these processes to an industrial level.
Low yields and the great difficulty in finding sufficiently selective catalysts to avoid the formation of numerous byproducts are the main challenges. Faced with this observation, an alternative emerges: using carbon monoxide (CO) as a reactant instead, a strategy that would likely optimize the conversion to more complex compounds.
© Marc Fontecave
In this context, a team from the Laboratory of Biological Process Chemistry (CNRS/Collège de France/Sorbonne Université) has designed, in partnership with TotalEnergies, an innovative catalyst based on copper nitride enriched with gold nanoparticles and isolated silver atoms.
Electrochemical tests revealed that this unique architecture promotes efficient conversion of CO into multicarbon alcohols, particularly ethanol and propanol, at the expense of ethylene formation, which is usually the main product of these reactions. Theoretical calculations show that gold and silver modify the electronic properties of copper, thus favoring reaction pathways leading to alcohols rather than hydrocarbons.
These results open interesting prospects for the sustainable production of fuels and chemical precursors from CO. In the long term, this technology published in Nature Materials could be integrated into industrial processes aimed at recycling CO emitted by certain industries, thereby reducing their overall carbon footprint while valorizing this underutilized gas.
Editor: AVR
Reference:
Incorporation of isolated Ag atoms and Au nanoparticles in copper nitride for selective CO electroreduction to multicarbon alcohols
Hong Phong Duong, Jose Guillermo Rivera de la Cruz, David Portehault, Andrea Zitolo, Jacques Louis, Sandrine Zanna, Quentin Arnoux, Moritz W. Schreiber, Nicolas Menguy, Ngoc-Huan Tran & Marc Fontecave.
Nature Materials 2025
https://doi.org/10.1038/s41563-025-02153-6