Follow us on Google News (click on ☆)

- DNA quadruplexes, a recent discovery with accelerating research. Why such enthusiasm for these structures formed by four DNA strands identified in over 700,000 sequences of our genome?
Researchers have speculated about the existence of these structures since the late 80s, but it wasn't until their demonstration in human cells in 2012 (1) that we began to better understand where, when, and how they form in our genome and what their functions could be. We discovered that quadruplexes can impact the genome in multiple ways: they act as genetic switches controlling the expression of certain genes; they can also hinder the work of enzymes that regulate DNA replication and transcription, thereby disrupting genomic stability. Cancer biologists are particularly interested in these properties: utilizing DNA quadruplexes as targets to slow the uncontrolled division of cancer cells by damaging their genome.
Alongside this, my research team has been interested in quadruplexes since 2019, not in tumor cells but in neuronal ones (2). Quite unexpectedly, we found that the number of DNA quadruplexes in these cells increases with age, leading to a series of dysfunctions. Could this be a molecular basis of aging? This deserved further investigation but convincing neurobiologists of the possible role played by DNA quadruplexes in neuronal cells and in their aging was no easy task. In collaboration with A. Tsvetkov's group in Houston (USA), we showed that quadruplexes indeed play a role in this aging, and we go even further by suggesting that the molecules stabilizing DNA quadruplexes to curb cancer cell proliferation also damage the genome of central nervous system cells and accelerate their aging.
- Until now, scientists have sought to stabilize DNA quadruplexes to slow tumor proliferation. How to destabilize them to counter the aging of neuronal cells?
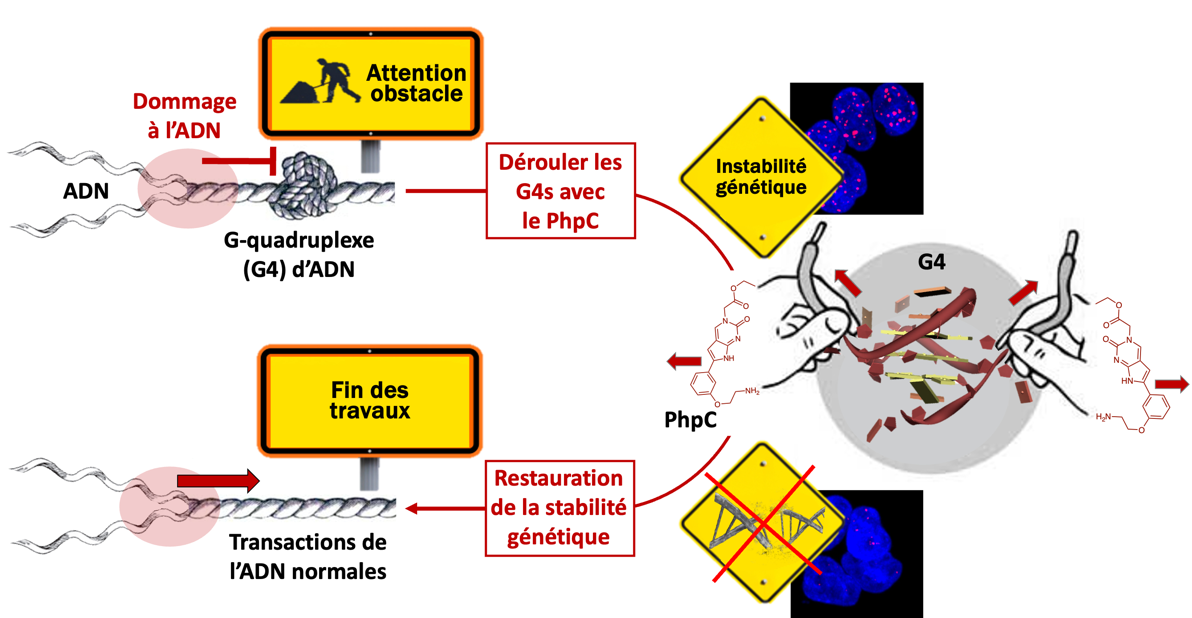
The situation is indeed complex on several levels. First, because the work conducted over the past 20 years has focused on stabilizing quadruplexes with small molecules (or ligands). When we began this work, neither a validated destabilizing agent prototype nor tests to identify them existed. There was everything to do. An additional strategic difficulty also came from the fact that we were looking to interact with quadruplexes to eliminate them, which meant following and interpreting the disappearance of the signal characterizing them during our measurements. This disappearance could be multifactorial and thus subject to misinterpretation, so our approach had to be rigorous and cautious.
In 2021 (3), we validated a series of in vitro tests to identify small molecules capable of destabilizing quadruplexes. This work led to the identification of a very promising prototype, a molecule named PhpC, which has very recently been tested on human cells. (4) Through specific techniques of optical imaging and affinity precipitation, we were able to show that PhpC is indeed capable of reducing the number of quadruplexes. Although the effects observed are weak, they are significant. We are now working to improve the efficiency of this family of molecules. Alas, we encounter difficulties in funding our research, which aims to decipher and understand the mechanism of action of this entirely new class of compounds. Perhaps because this approach implies a paradigm shift...
- What could be the impacts of your work in the field of neurobiology, in the short, long, or even very long term?
The applications and implications of this work are potentially huge. In the short term, these molecular tools (PhpC and its derivatives) can find applications in fundamental research related to all genetic diseases involving a quadruplex (cancers, neuropathologies, etc.). In the medium term, we will characterize the properties of these molecules in vivo by studying their stability (metabolization), bioavailability, their ability to modulate quadruplexes in animal models, etc. In the long or very long term, we hope to demonstrate the genoprotective properties of these compounds, their ability to bend the curve of neuronal aging, and slow the onset of age-related diseases.
Our motivation? The lack of effective treatment for these pathologies. The agents currently used in the clinic indeed act on the symptoms of these conditions but do not treat their causes because they are difficult to demonstrate. With the identification of the role of quadruplexes in these neuronal disorders, we may hold a promising therapeutic avenue that now needs to be validated. We know the road ahead of us is long and winding but the societal stakes are such that we cannot but try.
© David Monchaud
(*) DNA quadruplexes are unusual nucleic acid structures that form transiently in our genome and participate in key cellular life events. Made up of 4 DNA strands, these structures are used as targets to attempt to control the expression of the genes to which they belong.
Writer: CCdM
References:
(1) CNRS News (see: https://bit.ly/2UHpZjP)
(2) Small-molecule G-quadruplex stabilizers reveal a novel pathway of autophagy regulation in neurons, J. F. Moruno-Manchon et al., eLife 2020, 9, e52283 (https://doi.org/10.7554/eLife.52283).
- CNRS (https://bit.ly/39qtgrQ) and Research uB (https://bit.ly/2vABBKI)
- Sciences & Future (https://bit.ly/2SH2WD2)
- For Science (https://bit.ly/2XpbiBG)
- Swiss Radio Television (https://bit.ly/3byiHoF)
(3) Identifying G-quadruplex-DNA-disrupting small molecules, J. Mitteaux et al., J. Am. Chem. Soc. 2021, 143, 12567 (https://doi.org/10.1021/jacs.1c04426)
- CNRS (https://tinyurl.com/3s2pxdny)
(4) PhpC modulates G-quadruplex-RNA landscapes in human cells, J. Mitteaux et al., Chem. Commun. 2024,60, 424-427.