Follow us on Google News (click on ☆)
A team from the Laboratoire Matière et Systèmes Complexes and Flinders University (Australia) has shown that the mechanisms for detecting forces differ significantly from those usually involved in transmitting neuronal electrical signals. Published in the Journal of Experimental Physiology, this work highlights a new particularity of the enteric nervous system.
Illustrative image Pixabay
The only organ in our body with an intrinsic innervation, the intestine has one hundred million neurons connected to the central nervous system by only two thousand axons. This enteric nervous system detects the mechanical pressure exerted by the food bolus to command the muscles of the intestine for the next digestive actions: transport, mixing, or rejection.
Better understanding how the intestine detects these mechanical stimulations would shed light on the regulation of digestive motility and thus on associated pathologies, such as irritable bowel syndrome, which affects 10% of the population. The electrical activity of neurons depends on sodium channels, which allow the propagation of action potentials responsible for the nerve impulse.
Researchers from the Laboratoire Matière et Systèmes Complexes (MSC, CNRS/University of Paris) and Flinders University (Australia) have discovered that the neurons of the intestine exhibit mechanosensitive properties from their differentiation, but they do not detect mechanical pressure through action potentials.
To study this, scientists used intestines from genetically modified mice so that their cells would fluoresce depending on their electrical activation. The samples were taken during a critical phase of embryonic development when digestive motility transitions from purely muscular activity to activity modulated by the enteric nervous system. The neurons fluoresced during mechanical stimulations, indicating they were already mechanosensitive.
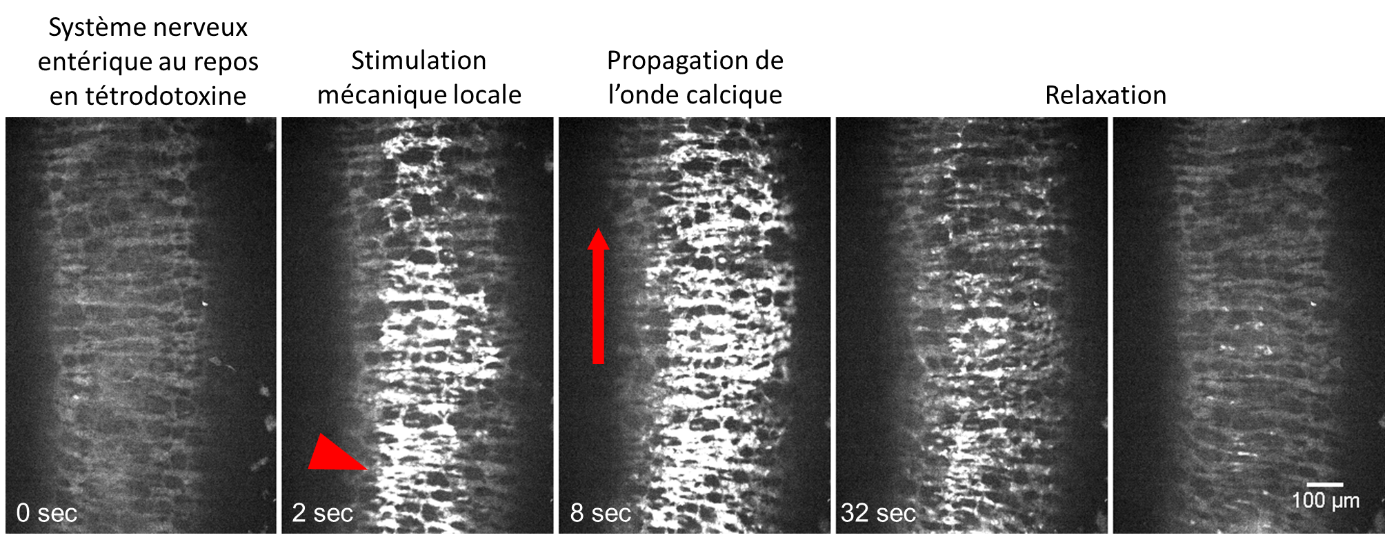
Still images during mechanical stimulation of the mouse intestine nervous system, maintained in tetrodotoxin. The fluorescence corresponds to the level of calcium inside the neurons, indicative of the propagation of an electrical signal.
© Amedzrovi Agbesi et al.
The team then injected tetrodotoxin, derived from the pufferfish highly prized in Japan under the name fugu, which blocks sodium channels. These molecules, present in the cell membrane, control the exchange of sodium ions between the inside of the cells and the external environment. While tetrodotoxin has long been known to completely halt action potentials and neuronal function, the mechanosensitive response was not affected at all.
The remarkable resistance of this response to a whole battery of ion channel inhibitors, beyond just sodium channels, suggests that it could largely be an intracellular phenomenon, relying on the disruption of calcium reservoirs inside the cell during mechanical stimulation.
However, the authors are not yet certain this explains the entire phenomenon and plan to continue their investigations. They also found that calcium channels play a very important role in generating spontaneous electrical signals in intestinal neurons.
According to well-established practice, researchers studying the enteric nervous system often use calcium channel inhibitors, which block the natural contractions of the intestine and thus facilitate imaging. This work suggests that this common practice might actually distort observations by inhibiting more functions than expected.
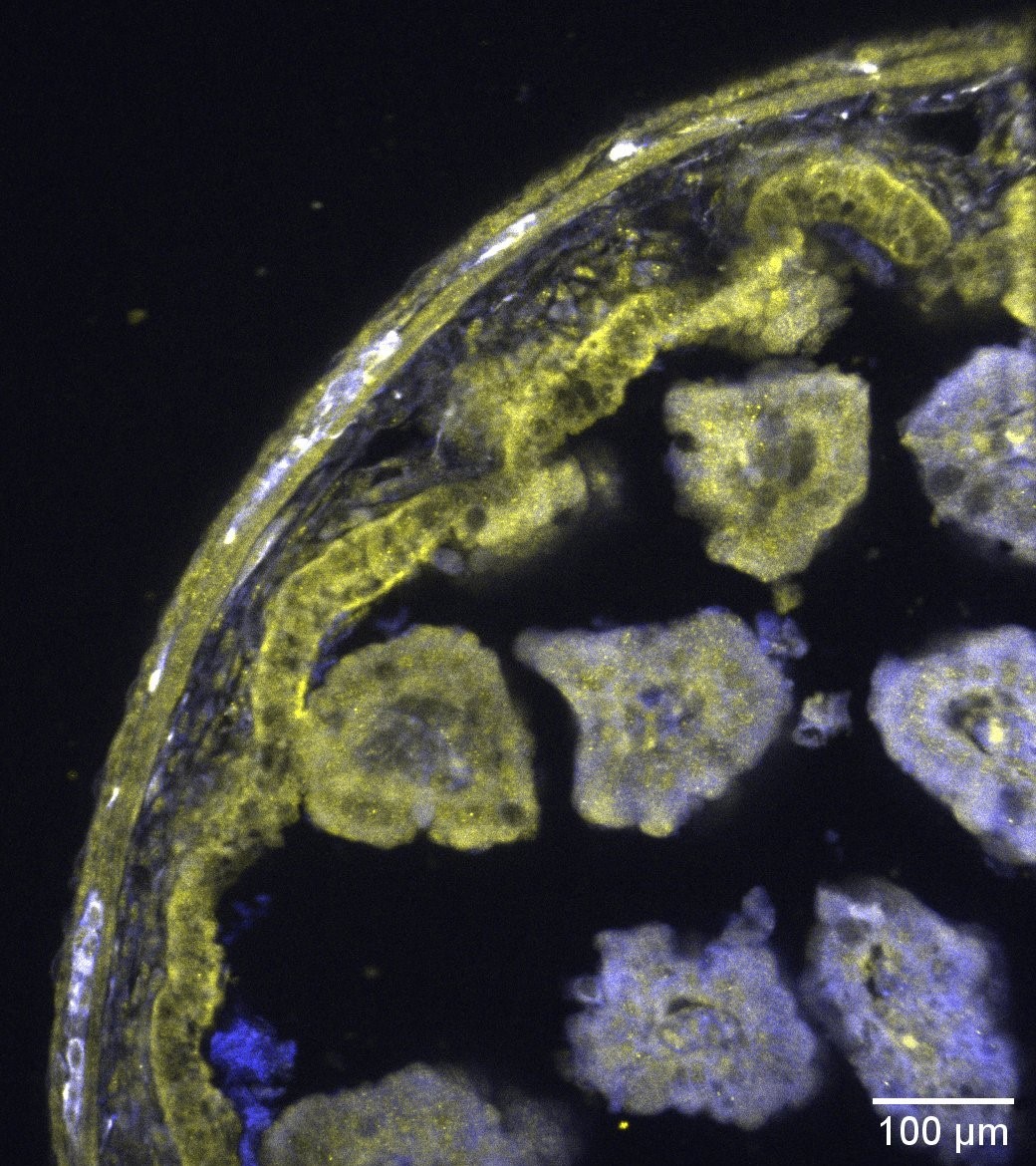
The white areas indicate the colocalization of neurons (blue) with L-type calcium channels (yellow) in this section of the mouse intestine.
© Amedzrovi Agbesi et al.
References:
Tetrodotoxin-resistant mechano-sensitivity and L-type calcium channel-mediated spontaneous calcium activity in enteric neurons.
Richard J. Amedzrovi Agbesi, Amira El Merhie, Nick J. Spencer, Tim Hibberd, and Nicolas R. Chevalier.
Journal of Experimental Physiology, 2024.
https://doi.org/10.1113/EP091977.