Follow us on Google News (click on ☆)

Gold nugget.
Illustration image Pixabay
When one tectonic plate subducts beneath another, it generates magmas rich in volatile substances such as water, sulfur, and chlorine. Along their path to the surface, these magmas release magmatic fluids in which sulfur and chlorine bind to metals, such as gold and copper, and transport these metals to the Earth's surface. Since the extreme conditions of natural magmas are very difficult to reproduce in the laboratory, the precise role of different forms of sulfur in metal transport remains highly debated.
An innovative approach by a team from the University of Geneva (UNIGE) demonstrates that sulfur, in its bisulfide form (HS-), is crucial for the transport of gold in magmatic fluids. These results are published in Nature Geoscience.
When two tectonic plates converge, the subducting plate plunges into the Earth's mantle, heats up, and releases a large amount of water. This water lowers the melting temperature of the mantle, which melts under high pressure and at temperatures above a thousand degrees Celsius to generate magmas. Since liquid magma is less dense than the rest of the mantle, it then migrates toward the Earth's surface.
Using a cutting-edge methodology, the UNIGE team was able to show that bisulfide is responsible for transporting the majority of gold.

The Solfatara on the island of Vulcano, near the Sicilian coast, with elemental sulfur precipitated from volcanic gases originating from magmatic fluids.
© Zoltán Zajacz
"Due to the drop in pressure, magmas rising toward the Earth's surface become saturated with a water-rich fluid, which is then released as bubbles of magmatic fluid," explains Stefan Farsang, a postdoctoral researcher at the Department of Earth Sciences at the Faculty of Sciences of UNIGE and the first author of the study. Magmatic fluids are thus composed partly of water but also of dissolved volatile elements such as sulfur and chlorine. These two elements are crucial because they extract gold, copper, and other metals from the silicate liquid into the magmatic fluid, thereby facilitating their migration to the surface.
Multiple forms of sulfur
Sulfur can easily be reduced or oxidized, meaning it can lose or gain electrons, a process called "redox." The redox states of sulfur are important because they influence its ability to bind to other elements, such as metals. However, a debate has divided the scientific community for over a decade: what is the redox state of sulfur present in the magmatic fluid that mobilizes and transports metals?
Zoltán Zajacz, associate professor at the Department of Earth Sciences at the Faculty of Sciences of UNIGE and co-lead author of the study, recounts: "A seminal paper in 2011 had suggested that sulfur radicals S3- played this role. However, the experimental and analytical methods had several limitations, particularly when it came to reproducing the relevant magmatic pressure-temperature and redox conditions, which we have now overcome."
Methodological revolution
The UNIGE team placed a quartz cylinder and a liquid with a composition similar to magmatic fluid in a hermetically sealed gold capsule. The capsule was then placed in a pressure vessel, which was brought to the pressure and temperature conditions characteristic of magmas emplaced in the Earth's upper crust. "Above all, our setup allows flexible control of the redox conditions in the system, which was not possible before," adds Stefan Farsang.
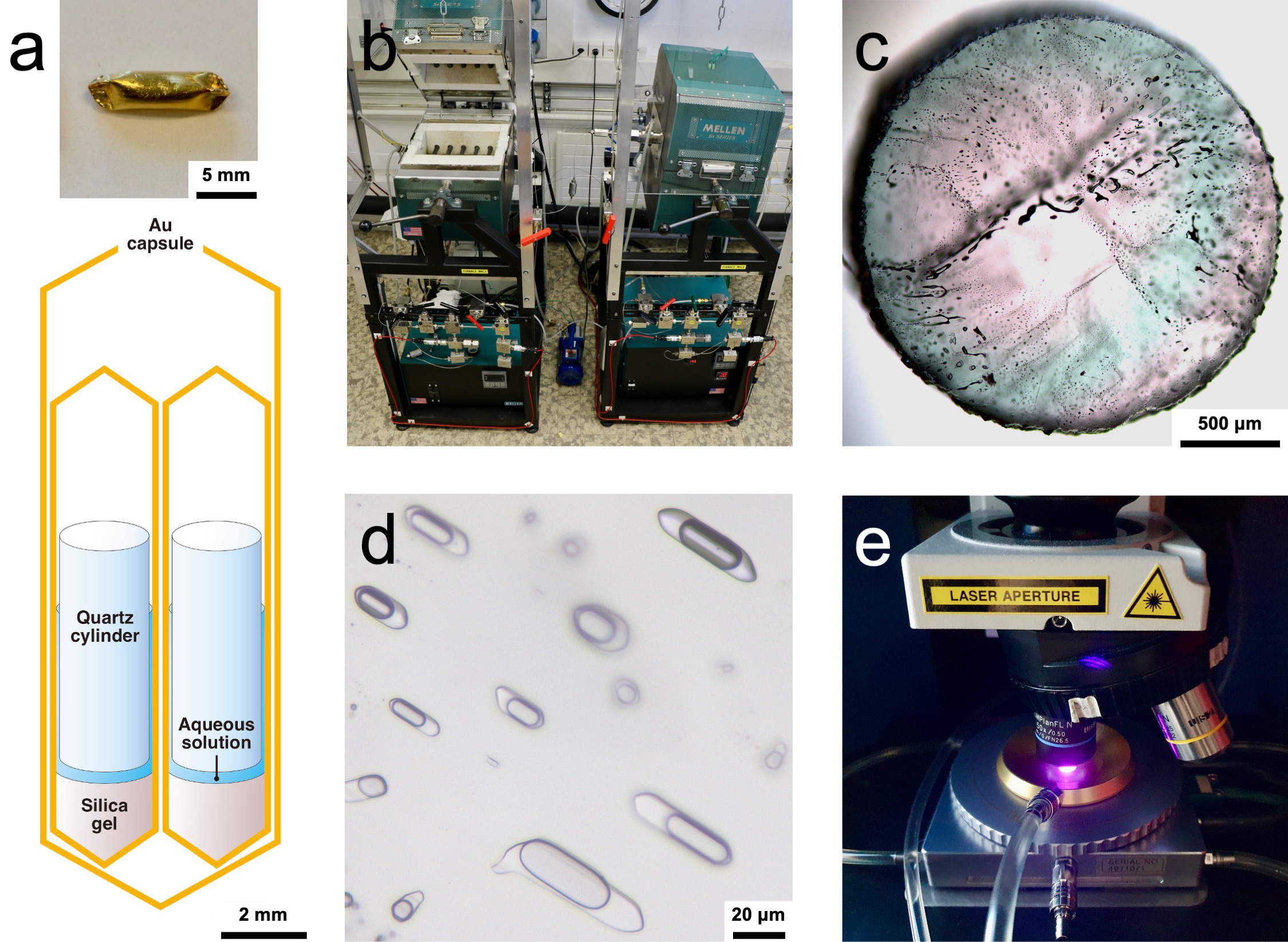
The device used by the UNIGE team: (a) a gold capsule containing an experimental fluid and a quartz cylinder, (b) prototypes of MHC pressure vessels; (c) a polished quartz disk with synthetic fluid inclusions, (d) a detailed image of these inclusions; (e) Raman analysis of the inclusions performed with the 405 nm laser in a heating stage.
© Stefan Farsang and Zoltan Zajacz - 'Sulfur species and gold transport in arc magmatic fluids', Nature Geoscience, December 2024.
During the experiments, the quartz cylinder is fractured, allowing the synthetic magmatic fluid to penetrate the fractures. The quartz then traps microdroplets of fluid, like those found in nature, and the form of sulfur they contain can be analyzed at high temperature and high pressure using lasers with an analytical technique known as Raman spectroscopy. While previous spectroscopic experiments were typically conducted up to 700 °C, the UNIGE team succeeded in increasing the temperature to 875 °C, characteristic of natural magmas.
Bisulfide as a transporter
The study shows that bisulfide (HS-), hydrogen sulfide (H₂S), and sulfur dioxide (SO₂) are the main sulfur species present in the experimental fluids at magmatic temperatures. The role of bisulfide in metal transport has already been well documented in lower-temperature "hydrothermal" fluids, which originate from higher-temperature magmatic fluids.
However, it was thought that bisulfide had very limited stability at magmatic temperatures. Thanks to a cutting-edge methodology, the UNIGE team was able to demonstrate that, in magmatic fluids as well, bisulfide is responsible for transporting the majority of gold.
"By carefully selecting laser wavelengths, we also showed that in previous studies, the amount of sulfur radicals in geological fluids was greatly overestimated and that the results of the 2011 study were actually based on a measurement artifact, thus putting an end to this debate," says Stefan Farsang. The natural conditions leading to the formation of significant precious metal deposits are now clarified.
Given that a large portion of global copper and gold production comes from deposits formed by magmatic fluids, this study can contribute to their exploration by opening important perspectives for understanding their formation.