Follow us on Google News (click on ☆)
The study, published in ACS Nano, reveals a previously unknown mechanism. Small clusters of the alpha-synuclein protein, called oligomers, dynamically perforate the membrane of neurons. This phenomenon is thought to disrupt the internal chemical balance of the cells, leading to their progressive degeneration.
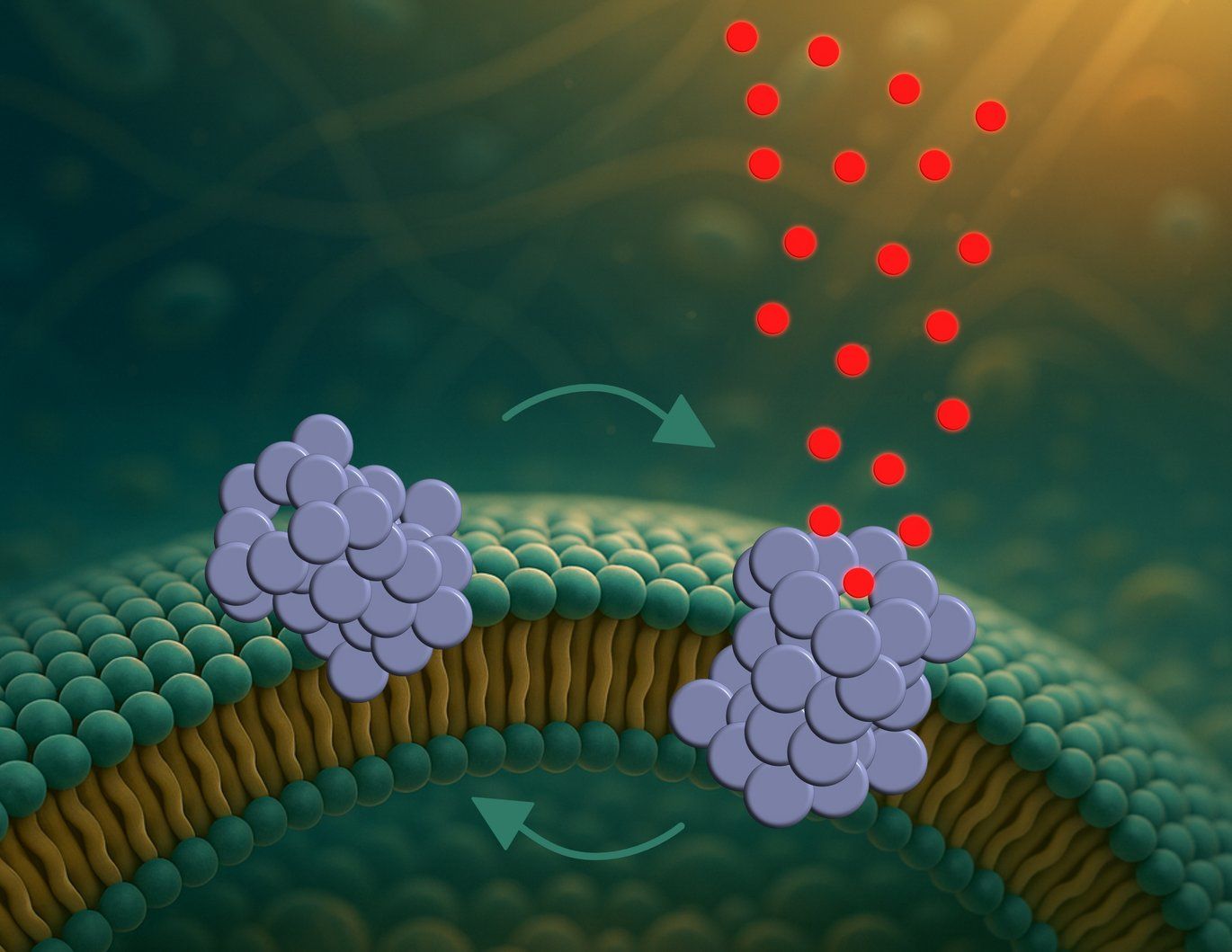
The image shows an α-synuclein oligomer (blue) partially inserted into a cell membrane (left).
Over time, it forms a pore (right) that allows molecules to pass through for a short period. The oligomer then returns to its initial state and undergoes a dynamic transition between the two states.
Image: Mette Galsgaard Malle - Aarhus University
A three-step mechanism observed for the first time
The scientists used synthetic vesicles to model neuronal membranes. This innovative platform allowed them to observe interactions at the molecular scale. They were able to track the behavior of the proteins in real time.
The infiltration process occurs in three distinct phases. The oligomers first attach to the surface of the cell membrane. They then partially embed themselves into it before organizing into pore-like structures. These openings then allow substances to pass through in an uncontrolled manner.
The major novelty lies in the dynamics of these pores. The team found that they do not remain permanently open. They open and close randomly, like microscopic revolving doors. This direct observation was unprecedented.
Progressive toxicity opening therapeutic avenues
This intermittent nature of the pores may explain the slow progression of the disease. A permanent opening would lead to rapid cell death. The dynamic nature of the phenomenon allows the cell's regulatory systems to temporarily compensate for the leaks.
Membranes with high curvature are the most sensitive. Mitochondria, the energy powerhouses of cells, therefore appear to be particularly vulnerable. This discovery could guide future treatment strategies towards protecting these organelles.
Nanobodies, antibody fragments, were tested to neutralize the oligomers. They proved effective for detection but not for blocking pore formation. The next step will be to validate these results in more complex biological models.
To go further: What is the normal role of alpha-synuclein?
In a healthy state, alpha-synuclein is an abundant protein in nerve terminals. It participates in the proper course of communication between neurons. Its main action concerns the release of neurotransmitters.
It interacts with synaptic vesicles, small sacs containing chemical messengers. The protein facilitates the fusion of these vesicles with the cell membrane. This fusion is essential for transmitting the nerve impulse.
Its misfolding leads to a loss of its beneficial function. The protein then becomes incapable of fulfilling its role in the synapse. This dual toxicity, loss of function and toxic gain of function, worsens the neurodegenerative process.