Follow us on Google News (click on ☆)
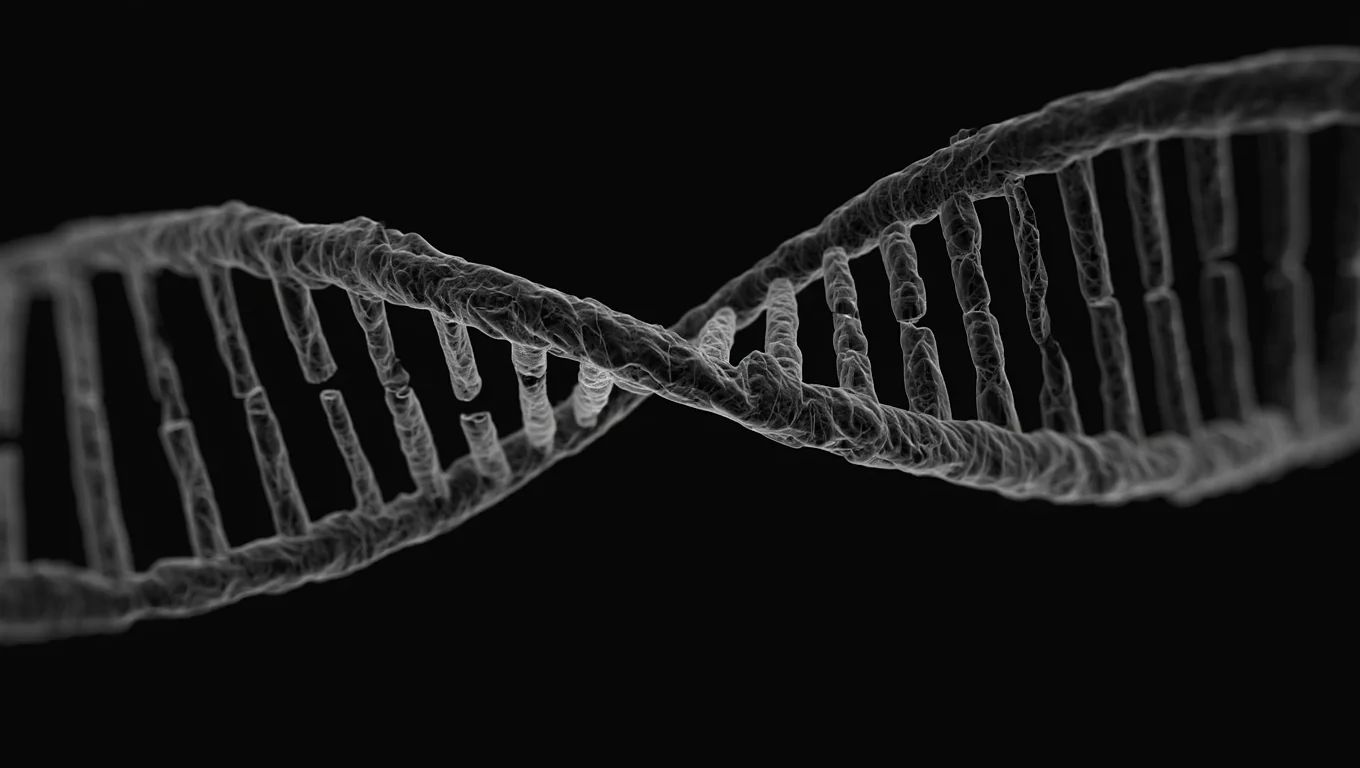
The international team used cryo-electron microscopy to observe a helicase, an enzyme essential for DNA replication. This work reveals a mechanism far more subtle than previously imagined, with potential implications for treating viral infections and cancers.
The unprecedented functioning of helicase
Contrary to previous hypotheses, helicase doesn't instantly separate DNA strands. Instead, it acts like a six-piston molecular motor, consuming ATP to gradually release tension between the strands.
This mechanism allows for natural DNA unwinding, comparable to releasing a compressed spring. Researchers also discovered that two helicases cooperate to form "replication forks," ensuring simultaneous copying of both strands.
The study shows this process is universal, from viruses to humans. This evolutionary conservation suggests that specifically blocking viral helicase could be a promising antiviral strategy, particularly against cancer-causing papillomaviruses.
Medical and technological applications
Viruses like smallpox and certain cancers use helicases with the same mechanism as human cells. By precisely targeting their function, treatments could be developed to block their replication without affecting healthy DNA.
Beyond medicine, this discovery inspires the design of synthetic nanomachines. Their energy efficiency, based on similar principles, could revolutionize molecular-scale technologies.
Thanks to cryo-electron microscopy, this study marks a key milestone in structural biology. It demonstrates how cutting-edge tools now allow visualization of cellular processes with unmatched precision.
Going further: How does ATP power molecular machines?
Adenosine triphosphate (ATP) acts as the universal "energy currency" of cells. Its chemical structure contains high-energy phosphate bonds that release about 7.3 kcal/mol when broken. This energy is used by motor proteins like helicases to perform mechanical work at the nanoscale.
When ATP binds to helicase, it induces a conformational change in the enzyme. This movement is amplified by ATP hydrolysis into ADP (adenosine diphosphate), which releases an inorganic phosphate. Each ATP binding/hydrolysis/release cycle advances the helicase one "step" along the DNA, like a molecular piston.
This mechanism is remarkably efficient: a single ATP molecule separates 1 to 2 DNA base pairs. The six helicase subunits act in coordination, creating a rotational movement reminiscent of an internal combustion engine, but with atomic precision.
Biophysics studies show this system converts about 50% of chemical energy into mechanical work - efficiency far superior to macroscopic engines. This performance explains why this mechanism has been conserved by evolution from bacteria to humans.