This reactor transforms CO2 from the air into fuel using the Sun 🌍
Published by Cédric,
Article author: Cédric DEPOND
Source: Nature Energy
Other Languages: FR, DE, ES, PT
Article author: Cédric DEPOND
Source: Nature Energy
Other Languages: FR, DE, ES, PT
Follow us on Google News (click on ☆)
This technology, inspired by photosynthesis, could allow us to rethink our approach to energy production and the fight against climate change.
Unlike traditional carbon capture and storage methods, this reactor requires neither fossil fuels nor complex infrastructure to transport or store CO₂. It operates entirely on solar energy, transforming a greenhouse gas into a useful resource. This approach paves the way for a circular economy, where CO₂ becomes a raw material rather than waste.
A device inspired by nature
The solar reactor uses specialized filters to capture CO₂ from the air at night, like a sponge absorbing water. When the Sun rises, sunlight triggers a chemical reaction that converts the captured CO₂ into syngas, a gaseous mixture used to produce fuels and chemicals.
This process relies on a hybrid catalyst combining molecular and semiconductor materials, optimizing conversion efficiency. Unlike existing technologies, this system operates under mild conditions and does not require pure CO₂ or high temperatures.
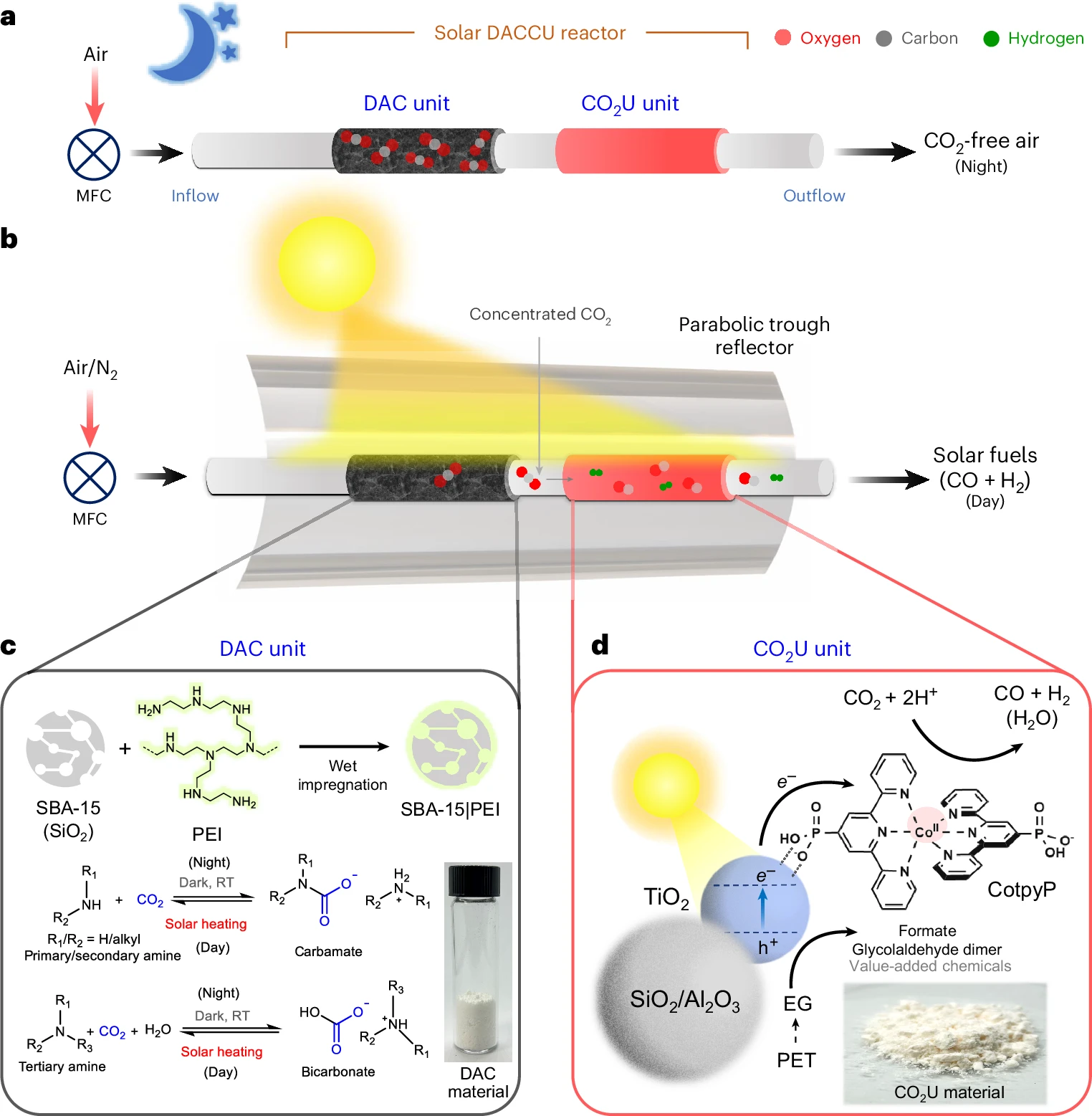
a) Diagram of the system operating at night without light.
b) Diagram of the overall system operating during the day with light.
c) Carbon capture unit with CO₂ capture and release equations.
d) Solar-powered CO₂U unit, material composition, and associated reduction and oxidation reactions.
By separating the capture and conversion steps, the researchers have solved a major problem: oxygen interference, which previously limited the efficiency of similar devices. This innovation enables faster and more efficient conversion of atmospheric CO₂.
Promising applications
This device could be used to produce clean fuels for cars, airplanes, or even essential chemicals, without emitting additional CO₂. It also offers a solution for remote areas where access to energy networks is limited.
The researchers envision decentralized production, allowing communities or individuals to generate their own fuel. This approach could reduce dependence on fossil fuels while contributing to the reduction of greenhouse gas emissions.
Finally, this technology could play a key role in the chemical and pharmaceutical industries, where syngas is an essential raw material. By using atmospheric CO₂, these sectors could reduce their carbon footprint while continuing to produce the products we rely on daily.
To go further: What is syngas?
Syngas (or "synthesis gas") is a gaseous mixture primarily composed of carbon monoxide (CO) and hydrogen (H₂). It is produced by the gasification of carbonaceous materials, such as coal, natural gas, or, in this case, carbon dioxide (CO₂). This gas is a key raw material in the chemical and energy industries, serving as the basis for the production of numerous products.
Historically, syngas has been used to produce liquid fuels via the Fischer-Tropsch process, which converts CO and H₂ into hydrocarbons. It is also used to manufacture ammonia, a key component of fertilizers, as well as methanol, an important solvent and chemical precursor. Its versatility makes it a cornerstone of modern industry.
In the context of the energy transition, syngas produced from CO₂ captured from the air represents a sustainable alternative to traditional methods, which rely on fossil fuels. By using renewable sources like solar energy for its production, the carbon footprint is reduced while creating a circular economy.
Finally, syngas could play a central role in decarbonizing sectors that are difficult to electrify, such as aviation or the production of complex chemicals. By combining CO₂ capture and conversion into syngas, this technology offers a promising path to reconcile industrial development with environmental preservation.