👽 This 'super alcohol' could be the key to life in the universe
Follow us on Google News (click on ☆)
Methanetetrol stands out due to its unique structure, with four oxygen-hydrogen groups around a single carbon atom. According to researchers, this molecule could play a key role in prebiotic chemistry, serving as a fundamental building block for life in the universe. The findings were published in Nature Communications, marking a significant breakthrough in our understanding of extraterrestrial chemical processes.
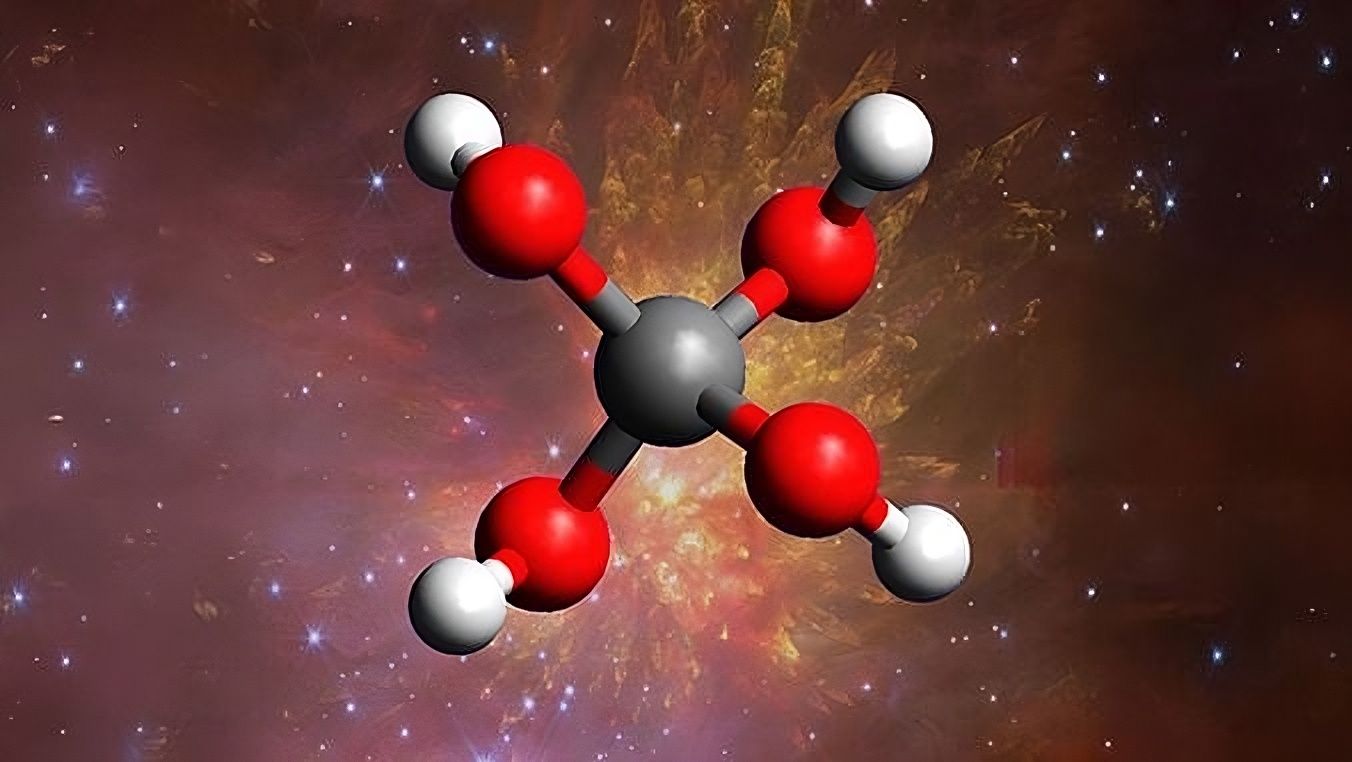
Artist's depiction of methanetetrol, a key molecule for understanding the origin of life in the universe.
Credit: Andrew Turner
To recreate the extreme conditions of space, scientists used a cryocooler at temperatures approaching -268 degrees Celsius (-450°F). They then exposed a mixture of water and carbon dioxide to radiation similar to cosmic rays. This experiment allowed them to observe the formation of methanetetrol in small quantities, confirming its stability in space-like environments.
The detection of methanetetrol was achieved using ultraviolet light, revealing its presence in gaseous form. This innovative method could be applied to the search for similar molecules in space.
The implications of this discovery are vast. Methanetetrol, when decomposing, can generate essential compounds for life, such as water and hydrogen peroxide. This property makes it an ideal candidate for studying the chemical mechanisms behind the origins of life, not only on Earth but also on other planets.
Researchers now plan to study how methanetetrol interacts with other molecules in interstellar dust clouds. These environments, where stars and planets form, could host the chemical reactions leading to life.
What is an ortho acid and why is it important for life?
Ortho acids are a class of chemical compounds characterized by multiple hydroxyl (-OH) groups attached to the same carbon atom. These molecules are particularly unstable, making them difficult to study in the lab.
Their instability is due to the strong repulsion between hydroxyl groups, which tend to separate to form more stable molecules. Despite this, ortho acids are considered key intermediates in prebiotic reactions, potentially leading to the formation of complex organic molecules.
In the case of methanetetrol, its unique structure makes it an ideal candidate for studying the earliest stages of life's chemistry. Its ability to generate essential compounds like water and hydrogen peroxide upon decomposition makes it a crucial element in the search for the origins of life.
This discovery paves the way for new research into the conditions necessary for the emergence of life, not only on Earth but also in other parts of the universe.