Follow us on Google News (click on ☆)
Glioblastoma is the most common and deadliest cancer of the adult central nervous system. Despite a standard care protocol established in 2005, combining maximum surgery followed by radio- and chemotherapy sessions, patients survive an average of only 15 months after their diagnosis. This aggressiveness is particularly linked to the presence of cancerous stem cells called glioblastoma stem-like cells, or GSCs. These cells, involved in the initiation, growth, and relapse of the tumor, represent therefore prime targets for the development of new therapeutic strategies.
Lysosomes Play a Critical Role in Maintaining Cancer Stem Cells
Lysosomes, cellular constituents, form a dynamic network of vesicles (small sacks), with an acidic pH, involved in the transportation of substances within the cell. They participate in metabolic processes and are responsible for digesting particles ingested by the cell and for degrading faulty cellular components.
Previous research shows that they also allow the survival of glioblastoma stem cells outside their protective niche within the tumor. Indeed, lysosomes extend growth factor signals and facilitate the spread and proliferation of cancer cells. In glioblastoma stem cells, altering the quantity and quality of lysosomes leads to specific death of these cells. Thus, lysosomes are a critical therapeutic target to influence the life-and-death decisions of these glioblastoma stem cells and thus control their population.
To destabilize the wall of the small sacks that lysosomes are, within glioblastoma stem cells, scientists have identified the role of the protease MALT1 (from the paracaspase family). Inhibiting this enzyme, recently defined as a crucial mediator of lysosome homeostasis, results in a lysosome-dependent death of glioblastoma stem cells. This, via a mechanism involving the mRNA-binding protein Quaking and the modulation of key protein expressions within the lysosomal compartment. However, the mechanisms of action of MALT1 remain poorly characterized.
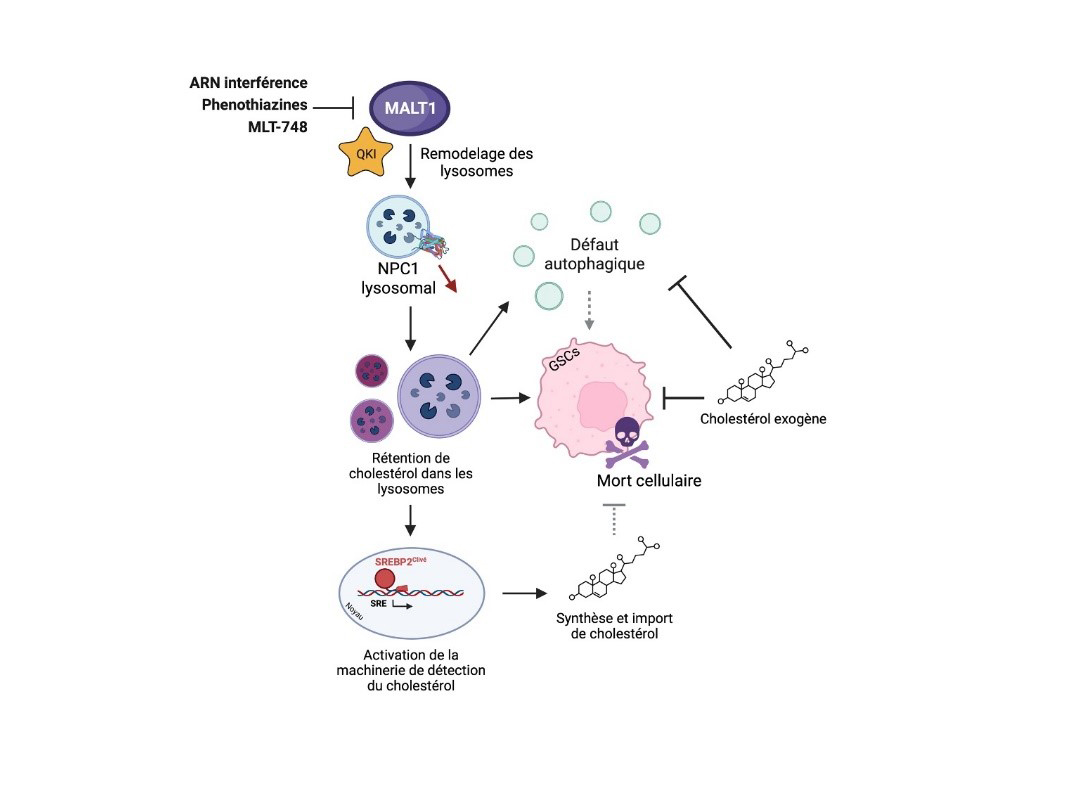
Figure: Model of MALT1 action in maintaining lysosomal homeostasis
The protease MALT1 modulates the lysosomal localization of the NPC1 protein and cholesterol homeostasis. In glioblastoma stem-like cells (GSCs), inhibiting MALT1 activity or interfering with its expression level remodels the lysosomal compartment, notably reducing the amount of the cholesterol transporter NPC1. This leads to intra-lysosomal retention of cholesterol, inducing a defect in autophagic degradation, activation of the machinery allowing the import and synthesis of cholesterol, and ultimately, cell death of GSCs. Adding exogenous cholesterol to GSCs with inhibited MALT1 activity partially counteracts the mentioned phenotypes, positioning thus the lysosomal transport of cholesterol as a target of GSCs.
© Clément Maghe.
A Flood of Lysosomal Cholesterol to Destroy Glioblastoma Stem Cells
The results of this study provide a better understanding of the system's workings. Indeed, they suggest that the repression of MALT1, through pharmacological agents or by RNA interference (a technique that allows for the specific inhibition of protein synthesis by destroying its corresponding messenger), modifies the homeostasis (the balance between the inside and the outside) of cholesterol. Cholesterol then accumulates in the vesicles of the lysosomal and late endosomal compartment. This failure of cholesterol supply leads to cell death and autophagy defects (the cell's self-cleaning system), which can be partially reversed by providing glioblastoma stem cells with membrane-permeable cholesterol.
These results were obtained through the combination of RNA sequencing (RNA-seq) analysis and proteome quantification, conducted on patient-derived glioblastoma stem cells subjected to a pharmacological inhibitor of MALT1 proteolytic activity.
Molecularly, a targeted proteome analysis of lysosomes revealed that lysosomal cholesterol transporters of the Niemann-Pick C (NPC) type are less present when MALT1 activity is hindered. In line with these data, pharmacologically blocking or knocking down the expression of these NPC1/2 transporters replicates the effects of MALT1 loss-of-function, suggesting a similar action of these two molecules. Finally, inhibiting MALT1 or NPC1/2 slows tumor growth in immunodeficient mouse models carrying patient-derived glioblastoma stem cells.
This work has thus allowed the mapping of subcellular events participating in lysosomal destabilization induced by the molecular and pharmacological targeting of the paracaspase MALT1. These data bring forth the idea that the properties and maintenance of glioblastoma stem cells rely on lysosomal cholesterol homeostasis.
Reference:
The paracaspase MALT1 controls cholesterol homeostasis in glioblastoma stem-like cells through lysosome proteome shaping.
Maghe C, Trillet K, André-Grégoire G, Kerhervé M, Merlet L, Jacobs KA, Schauer K, Bidère N, Gavard J. Cell Rep. 2024 Jan 5;43(1):113631.
DOI: Cell