Follow us on Google News (click on ☆)
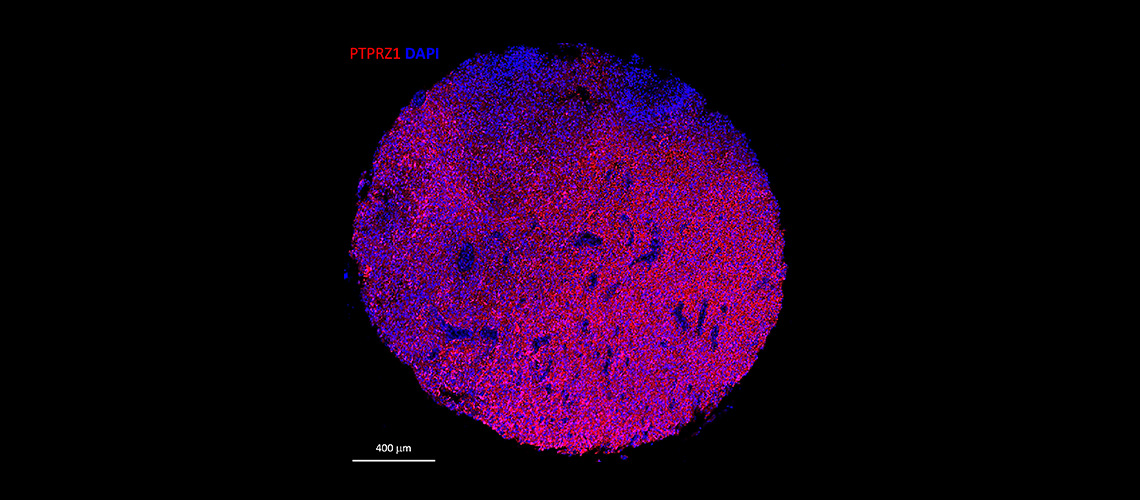
Immunofluorescence staining of a human glioblastoma tissue section. In red, PTPRZ1 markers, and in blue, cell nuclei (scale: 400 μm).
© Denis Migliorini - UNIGE/HUG
A team from the University of Geneva (UNIGE) and the Geneva University Hospitals (HUG) has managed to identify a specific marker on the surface of tumor cells and to generate immune cells equipped with an antibody to destroy them. Additionally, these cells, known as "CAR-T" cells, appear capable of targeting, within the tumor, diseased cells lacking this antigen while sparing healthy cells.
These findings, available in the journal Cancer Immunology Research, represent a first step toward the development of clinical trials in humans.
Glioblastomas have biological characteristics that make them particularly difficult to treat. Capable of inducing a microenvironment that limits the immune system's attack, they evade standard treatments and recur quickly.
Denis Migliorini, assistant professor in the Department of Medicine at the UNIGE Faculty of Medicine, holder of the ISREC Foundation Chair in Brain Tumor Immunology, member of the Center for Translational Research in Onco-Hematology (CRTOH), and associate physician in charge of the Neuro-Oncology Unit at HUG, is a specialist in "CAR-T cells", or chimeric antigen receptor T lymphocytes.
This immunotherapy involves collecting T lymphocytes — immune cells — from the patient, genetically modifying them in the laboratory to equip them with antibodies that can detect elements specific to tumor cells before reinjecting them so they can attack the tumor in a targeted manner.
“We have been working for several years to identify the protein markers expressed by the cells that make up these malignant gliomas,” explains Denis Migliorini. “One of these markers, PTPRZ1, proved particularly important: we were able to generate CAR-T cells carrying antibodies targeting PTPRZ1. This was a first step toward effective CAR-T against these tumors.”
mRNA to create a custom cell
Most CAR-T cells are produced using viral vectors, a technique that has proven successful in certain diseases but is less suitable for the brain. "They persist for a long time in blood cell cancers. However, the brain is a delicate organ, and such persistence could pose a risk of toxicity," explains Darel Martinez Bedoya, postdoctoral fellow in Denis Migliorini's laboratory and first author of this work.
The scientists instead introduced the desired antibody's messenger RNA into the T lymphocytes. The cellular machinery then produces the correct protein to create the receptor, which positions itself on the lymphocyte's surface and recognizes the tumor target.
“This technique offers many advantages. CAR-T provides a flexible platform: they allow multiple adaptations based on the specificity and evolution of the tumor,” Darel Martinez Bedoya explains.
Efficacy and safety
To ensure that the CAR-T cells only target tumor cells, the Geneva team first tested them in vitro on both healthy and diseased cells.
“We were pleasantly surprised to find that not only did CAR-T not attack healthy cells, but they were also able, through close proximity effects, to identify and fight diseased cells lacking the PTPRZ1 marker,” says Denis Migliorini. “In this context, CAR-T cells likely secrete pro-inflammatory molecules that contribute to the elimination of tumor cells, even in the absence of the original marker.”
The second step was to test the treatment in vivo using mouse models of glioblastoma. Tumor growth was controlled, significantly extending the lives of the mice without any signs of toxicity. “We administered the CAR-T directly into the tumor. This made it possible to use fewer cells and sharply reduce the risk of peripheral toxicity.
All indications are positive for now considering a first human clinical trial,” the scientists conclude.