Follow us on Google News (click on ☆)
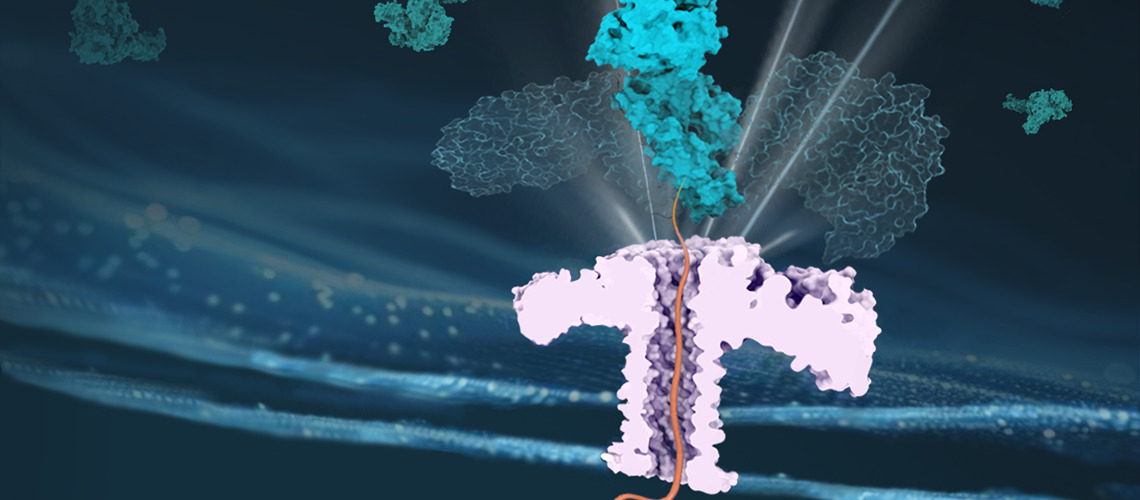
A chaperone protein facilitates the passage of another protein through a nanopore, thus ensuring its effective transport. © Cao - UNIGE
In animal and human cells, Hsp70 class chaperones are at the heart of this control system, overseeing a wide range of biological processes. Yet, despite their crucial role, their precise molecular mechanism remained elusive.
Using a cutting-edge nanopore technique, a team from the University of Geneva (UNIGE), in collaboration with EPFL, has made a significant breakthrough by determining how Hsp70s generate the force needed to manipulate the structure of their client proteins. These findings, which conclude a decade of debate, were published in Nature Communications.
To function correctly, proteins must fold into specific three-dimensional shapes. Chaperone proteins, such as Hsp70s, notably contribute to this folding process. To carry out these tasks, Hsp70s must forcefully manipulate protein structure, pulling them out of aggregates that spontaneously formed or passing them through narrow pores to direct them into key cellular compartments, such as mitochondria.
The team took advantage of nanopore technology to reproduce the in vivo configuration of protein translocation.
During the 1990s and early 2000s, the mechanism allowing Hsp70 chaperones to drive protein translocation was the subject of intense debate. Two main models were proposed based on different experiments, but no definitive answer was provided.
In 2006, a new theory, called Entropic Pulling, was proposed by Professor Paolo De Los Rios from EPFL and Professor Pierre Goloubinoff from the University of Lausanne (UNIL) and their collaborators. Entropic Pulling could explain all existing observations regarding protein translocation in mitochondria. It could also apply to other cellular functions of Hsp70, such as protein disaggregation.
Experimental evidence
Over the years, this theory has helped interpret an increasing number of results but had remained without direct experimental confirmation. The group led by Chan Cao, assistant professor in the Department of Analytical and Inorganic Chemistry at the Faculty of Science at UNIGE, specializes in single-molecule bioanalysis, particularly nanopore detection.
This approach consists of reading the ionic current response when a molecule passes through a nanometer-scale pore. The pore can be made of a biological protein embedded in a lipid membrane or in a fabricated solid-state material. The development of nanopore technology aims to create high-resolution sensors for the detection of target molecules in complex matrices and for polymer sequencing.
In this recent work, the team took advantage of nanopore technology to reproduce the in vivo configuration of protein translocation. Professor Chan Cao explains: “Our results provide clear evidence of the entropic pulling mechanism of Hsp70 chaperones, ruling out the two other previously proposed models, namely the Power Stroke and Brownian Ratchet models.”
A powerful force at the molecular level
In the Entropic Pulling mechanism, the chaperone protein, by pulling on the target protein, increases its movement possibilities, thus generating a so-called entropic force. Verena Rukes, doctorate student and lead author of the study, explains: “Our analysis estimated the entropic pulling force to be about 46 pN across a distance of 1 nm, indicating a remarkably strong force at the molecular level.”
Paolo De Los Rios, from the Institute of Physics and the Institute of Bioengineering at EPFL, adds: “The theory we proposed in 2006 accounted for most of the physics of the system comprising Hsp70, the translocating protein, and the translocation pore, but it remained a theory, even if it indirectly matched most observations.
Thanks to the outstanding work of Professor Chan Cao and her team, we now have direct evidence of this theory and, most importantly, a quantitative estimate of its force. It turns out to be remarkably high, which explains why Hsp70s are so effective at modifying the structure of their target proteins.”
Moreover, this work establishes nanopore detection as a powerful technique to explore the molecular mechanisms of protein action.