Alzheimer: This Therapy Can Repair Patients' Synapses and Restore Their Memory
Published by Cédric,
Article Author: Cédric DEPOND
Source: The Journal of Clinical Investigation
Other Languages: FR, DE, ES, PT
Article Author: Cédric DEPOND
Source: The Journal of Clinical Investigation
Other Languages: FR, DE, ES, PT
Follow us on Google News (click on ☆)

Illustration Image Pixabay
In a recent study published in the February 1st issue of The Journal of Clinical Investigation, Buck Institute's associate professor, Tara Tracy, emphasizes the significance of this new approach. Instead of solely targeting toxic proteins like tau and beta-amyloid, responsible for plaque accumulation in the brains of Alzheimer's patients, Tracy's team investigated the role of a protein called KIBRA, present at the synapses.
Researchers found that KIBRA levels were significantly reduced in the brains of individuals with Alzheimer's, suggesting a close link between this protein and the impaired synaptic function associated with the disease. By studying laboratory mice with a condition similar to human Alzheimer's, the team observed that manipulating the KIBRA protein could reverse the observed memory deficits.
Indeed, KIBRA appears to play a key role in promoting synapse resilience, thus facilitating the restoration of their normal function. This discovery opens new therapeutic avenues, as explained by Grant Kauwe, another researcher at the Buck Institute. The findings suggest that KIBRA could not only serve as a biomarker to diagnose and monitor the disease's progression but also as a potential target for new therapies designed to restore memory in Alzheimer's patients.
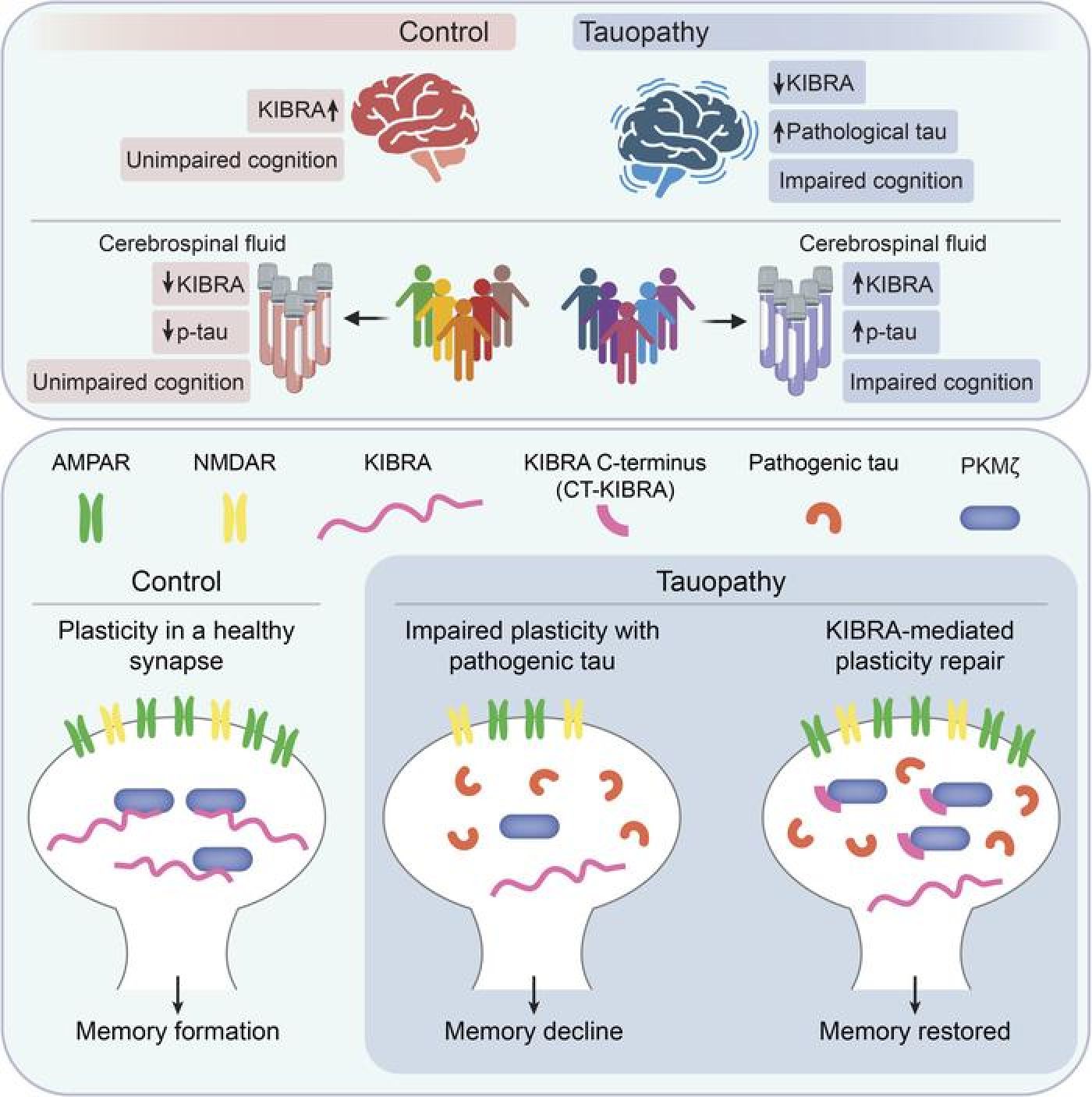
This innovative approach highlights the importance of diversifying therapeutic strategies against Alzheimer's disease. While current treatments mainly focus on reducing toxic proteins, this study sheds light on the urgency of addressing the synaptic alterations underlying memory loss in this devastating disease.