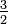Liste de potentiels standards - Définition
Source: Wikipédia sous licence CC-BY-SA 3.0.
La liste des auteurs de cet article est disponible ici.
La liste des auteurs de cet article est disponible ici.
La liste des potentiels standards, en volts, qui suit est relative à la tension obtenue avec l'électrode standard à hydrogène et est assemblée à partir de différents ouvrages.
Les valeurs sont obtenues sous ces conditions :
- température de 25 °C ;
- concentration effective à 1 mol/L pour chaque espèce aqueuse ou pour chaque espèce dans un amalgame de mercure ;
- pression partielle à 101,325 kPa (absolu) (1 atm ou 1,01325 bar) pour chaque réactif gazeux. Cette pression est utilisée, car la plupart des données disponibles l'utilisent au lieu de la plus moderne de 100 kPa.
- activité chimique de chaque substance pure, sous forme solide ou liquide, ou pour l'eau comme (solvant).
Le tableau peut être trié alphabétiquement en cliquant sur le bouton de tri.
Légende :
- (s) : solide ;
- (l) : liquide ;
- (g) : gaz ;
- (aq) : aqueux (défaut pour toutes les espèces ayant une charge électrique) ;
- (Hg) : amalgame de mercure.
| Demi-équation | E° (V) | Réf. |
|---|---|---|
|
| −3,09 | |
| Li+ + e− ⇄ Li(s) | −3,0401 | |
| N2(g) + 4 H2O + 2 e− ⇄ 2 NH2OH(aq) + 2 OH− | −3,04 | |
| Cs+ + e− ⇄ Cs(s) | −3,026 | |
| Rb+ + e− ⇄ Rb(s) | −2,98 | |
| K+ + e− ⇄ K(s) | −2,931 | |
| Ba2+ + 2 e− ⇄ Ba(s) | −2,912 | |
| La(OH)3(s) + 3 e− ⇄ La(s) + 3OH− | −2,90 | |
| Sr2+ + 2 e− ⇄ Sr(s) | −2,899 | |
| Ca2+ + 2 e− ⇄ Ca(s) | −2,868 | |
| Eu2+ + 2 e− ⇄ Eu(s) | −2,812 | |
| Ra2+ + 2 e− ⇄ Ra(s) | −2,8 | |
| Na+ + e− ⇄ Na(s) | −2,71 | |
| La3+ + 3 e− ⇄ La(s) | −2,379 | |
| Mg2+ + 2 e− ⇄ Mg(s) | −2,372 | |
| ZrO(OH)2(s) + H2O + 4 e− ⇄ Zr(s) + 4OH− | −2,36 | |
| Al(OH)4− + 3 e− ⇄ Al(s) + 4 OH− | −2,33 | |
| Al(OH)3/sub>(s) + 3 e− ⇄ Al(s) + 3OH− | −2,31 | |
| H2(g) + 2 e− ⇄ 2 H− | −2,25 | |
| Ac3+ + 3 e− ⇄ Ac(s) | −2,20 | |
| Be2+ + 2 e− ⇄ Be(s) | −1,85 | |
| U3+ + 3 e− ⇄ U(s) | −1,66 | |
| Al3+ + 3 e− ⇄ Al(s) | −1,66 | |
| Ti2+ + 2 e− ⇄ Ti(s) | −1,63 | |
| ZrO2(s) + 4 H+ + 4 e− ⇄ Zr(s) + 2 H2O | −1,553 | |
| Zr4+ + 4 e− ⇄ Zr(s) | −1,45 | |
| TiO(s) + 2 H+ + 2 e− ⇄ Ti(s) + H2O | −1,31 | |
| Ti2O3(s) + 2 H+ + 2 e− ⇄ 2 TiO(s) + H2O | −1,23 | |
| Ti3+ + 3 e− ⇄ Ti(s) | −1,21 | |
| Mn2+ + 2 e− ⇄ Mn(s) | −1,185 | |
| Te(s) + 2 e− ⇄ Te2− | −1,143 | |
| V2+ + 2 e− ⇄ V(s) | −1,13 | |
| Nb3+ + 3 e− ⇄ Nb(s) | −1,099 | |
| Sn(s) + 4 H+ + 4 e− ⇄ SnH4(g) | −1,07 | |
| SiO2(s) + 4 H+ + 4 e− ⇄ Si(s) + 2 H2O | −0,91 | |
| B(OH)3(aq) + 3 H+ + 3 e− ⇄ B(s) + 3 H2O | −0,89 | |
| TiO2+ + 2 H+ + 4 e− ⇄ Ti(s) + H2O | −0,86 | |
| Bi(s) + 3 H+ + 3 e− ⇄ BiH3 | −0,8 | |
| 2 H2O + 2 e− ⇄ H2(g) + 2 OH− | −0,8277 | |
| Zn2+ + 2 e− ⇄ Zn(Hg) | −0,7628 | |
| Zn2+ + 2 e− ⇄ Zn(s) | −0,7618 | |
| Ta2O5(s) + 10 H+ + 10 e− ⇄ 2 Ta(s) + 5 H2O | −0,75 | |
| Cr3+ + 3 e− ⇄ Cr(s) | −0,74 | |
| [Au(CN)2]− + e− ⇄ Au(s) + 2 CN− | −0,60 | |
| Ta3+ + 3 e− ⇄ Ta(s) | −0,6 | |
| PbO(s) + H2O + 2 e− ⇄ Pb(s) + 2 OH− | −0,58 | |
| 2 TiO2(s) + 2 H+ + 2 e− ⇄ Ti2O3(s) + H2O | −0,56 | |
| Ga3+ + 3 e− ⇄ Ga(s) | −0,53 | |
| U4+ + e− ⇄ U3+ | −0,52 | |
| H3PO2(aq) + H+ + e− ⇄ P(blanc) + 2 H2O | −0,508 | |
| H3PO3(aq) + 2 H+ + 2 e− ⇄ H3PO2(aq) + H2O | −0,499 | |
| H3PO3(aq) + 3 H+ + 3 e− ⇄ P(rouge) + 3H2O | −0,454 | |
| Fe2+ + 2 e− ⇄ Fe(s) | −0,44 | |
| 2 CO2(g) + 2 H+ + 2 e− ⇄ HOOCCOOH(aq) | −0,43 | |
| Cr3+ + e− ⇄ Cr2+ | −0,42 | |
| Cd2+ + 2 e− ⇄ Cd(s) | −0,40 | |
| GeO2(s) + 2 H+ + 2 e− ⇄ GeO(s) + H2O | −0,37 | |
| Cu2O(s) + H2O + 2 e− ⇄ 2 Cu(s) + 2 OH− | −0,360 | |
| PbSO4(s) + 2 e− ⇄ Pb(s) + SO42− | −0,3588 | |
| PbSO4(s) + 2 e− ⇄ Pb(Hg) + SO42− | −0,3505 | |
| Eu3+ + e− ⇄ Eu2+ | −0,35 | |
| In3+ + 3 e− ⇄ In(s) | −0,34 | |
| Tl+ + e− ⇄ Tl(s) | −0,34 | |
| Ge(s) + 4 H+ + 4 e− ⇄ GeH4(g) | −0,29 | |
| Co2+ + 2 e− ⇄ Co(s) | −0,28 | |
| H3PO4(aq) + 2 H+ + 2 e− ⇄ H3PO3(aq) + H2O | −0,276 | |
| V3+ + e− ⇄ V2+ | −0,26 | |
| Ni2+ + 2 e− ⇄ Ni(s) | −0,25 | |
| As(s) + 3 H+ + 3 e− ⇄ AsH3(g) | −0,23 | |
| MoO2(s) + 4 H+ + 4 e− ⇄ Mo(s) + 2 H2O | −0,15 | |
| Si(s) + 4 H+ + 4 e− ⇄ SiH4(g) | −0,14 | |
| Sn2+ + 2 e− ⇄ Sn(s) | −0,13 | |
| O2(g) + H+ + e− ⇄ HO2•(aq) | −0,13 | |
| Pb2+ + 2 e− ⇄ Pb(s) | −0,13 | |
| WO2(s) + 4 H+ + 4 e− ⇄ W(s) + 2 H2O | −0,12 | |
| P(rouge) + 3 H+ + 3 e− ⇄ PH3(g) | −0,111 | |
| CO2(g) + 2 H+ + 2 e− ⇄ HCOOH(aq) | −0,11 | |
| Se(s) + 2 H+ + 2 e− ⇄ H2Se(g) | −0,11 | |
| CO2(g) + 2 H+ + 2 e− ⇄ CO(g) + H2O | −0,11 | |
| SnO(s) + 2 H+ + 2 e− ⇄ Sn(s) + H2O | −0,10 | |
| SnO2(s) + 2 H+ + 2 e− ⇄ SnO(s) + H2O | −0,09 | |
| WO3(aq) + 6 H+ + 6 e− ⇄ W(s) + 3 H2O | −0,09 | |
| P(blanc) + 3 H+ + 3 e− ⇄ PH3(g) | −0,063 | |
| HCOOH(aq) + 2 H+ + 2 e− ⇄ HCHO(aq) + H2O | −0,03 | |
| 2 H+ + 2 e− ⇄ H2(g) | 0,0000 | ≡ 0 |
| S4O62− + 2 e− ⇄ 2 S2O32− | +0,08 | |
| Fe3O4(s) + 8 H+ + 8 e− ⇄ 3 Fe(s) + 4 H2O | +0,085 | |
| N2(g) + 2 H2O + 6H+ + 6 e− ⇄ 2 NH4OH(aq) | +0,092 | |
| HgO(s) + H2O + 2 e− ⇄ Hg(l) + 2 OH− | +0,0977 | |
| Cu(NH3)42+ + e− ⇄ Cu(NH3)2+ + 2 NH3 | +0,10 | |
| Ru(NH3)63+ + e− ⇄ Ru(NH3)62+ | +0,10 | |
| N2H4(aq) + 4 H2O + 2 e− ⇄ 2 NH4+ + 4 OH− | +0,11 | |
| H2MoO4(aq) + 6 H+ + 6 e− ⇄ Mo(s) + 4 H2O | +0,11 | |
| Ge4+ + 4 e− ⇄ Ge(s) | +0,12 | |
| C(s) + 4 H+ + 4 e− ⇄ CH4(g) | +0,13 | |
| HCHO(aq) + 2 H+ + 2 e− ⇄ CH3OH(aq) | +0,13 | |
| S(s) + 2 H+ + 2 e− ⇄ H2S(g) | +0,14 | |
| Sn4+ + 2 e− ⇄ Sn2+ | +0,15 | |
| Cu2+ + e− ⇄ Cu+ | +0,159 | |
| HSO4− + 3 H+ + 2 e− ⇄ SO2(aq) + 2 H2O | +0,16 | |
| UO22+ + e− ⇄ UO2+ | +0,163 | |
| SO42− + 4 H+ + 2 e− ⇄ SO2(aq) + 2 H2O | +0,17 | |
| TiO2+ + 2 H+ + e− ⇄ Ti3+ + H2O | +0,19 | |
| Bi3+ + 2e− ⇄ Bi+ | +0,2 | |
| SbO+ + 2 H+ + 3 e− ⇄ Sb(s) + H2O | +0,20 | |
| H3AsO3(aq) + 3 H+ + 3 e− ⇄ As(s) + 3 H2O | +0,24 | |
| GeO(s) + 2 H+ + 2 e− ⇄ Ge(s) + H2O | +0,26 | |
| UO2+ + 4 H+ + e− ⇄ U4+ + 2 H2O | +0,273 | |
| Re3+ + 3 e− ⇄ Re(s) | +0,300 | |
| Bi3+ + 3 e− ⇄ Bi(s) | +0,32 | |
| VO2+ + 2 H+ + e− ⇄ V3+ + H2O | +0,34 | |
| Cu2+ + 2 e− ⇄ Cu(s) | +0,340 | |
| [Fe(CN)6]3− + e− ⇄ [Fe(CN)6]4− | +0,36 | |
| O2(g) + 2 H2O + 4 e− ⇄ 4 OH−(aq) | +0,40 | |
| H2MoO4 + 6 H+ + 3 e− ⇄ Mo3+ + 2 H2O | +0,43 | |
| Bi+ + e− ⇄ Bi(s) | +0,50 | |
| CH3OH(aq) + 2 H+ + 2 e− ⇄ CH4(g) + H2O | +0,50 | |
| SO2(aq) + 4 H+ + 4 e− ⇄ S(s) + 2 H2O | +0,50 | |
| Cu+ + e− ⇄ Cu(s) | +0,520 | |
| CO(g) + 2 H+ + 2 e− ⇄ C(s) + H2O | +0,52 | |
| I2(s) + 2 e− ⇄ 2 I− | +0,54 | |
| I3− + 2 e− ⇄ 3 I− | +0,53 | |
| [AuI4]− + 3 e− ⇄ Au(s) + 4 I− | +0,56 | |
| H3AsO4(aq) + 2 H+ + 2 e− ⇄ H3AsO3(aq) + H2O | +0,56 | |
| [AuI2]− + e− ⇄ Au(s) + 2 I− | +0,58 | |
| MnO4− + 2 H2O + 3 e− ⇄ MnO2(s) + 4 OH− | +0,59 | |
| S2O32 − + 6 H+ + 4 e− ⇄ 2 S(s) + 3 H2O | +0,60 | |
| H2MoO4(aq) + 2 H+ + 2 e− ⇄ MoO2(s) + 2 H2O | +0,65 | |
| O2(g) + 2 H+ + 2 e− ⇄ H2O2(aq) | +0,70 | |
| Tl3+ + 3 e− ⇄ Tl(s) | +0,72 | |
| PtCl62− + 2 e− ⇄ PtCl42− + 2 Cl− | +0,726 | |
| H2SeO3(aq) + 4 H+ + 4 e− ⇄ Se(s) + 3 H2O | +0,74 | |
| PtCl42− + 2 e− ⇄ Pt(s) + 4 Cl− | +0,758 | |
| Fe3+ + e− ⇄ Fe2+ | +0,77 | |
| Ag+ + e− ⇄ Ag(s) | +0,7996 | |
| Hg22+ + 2 e− ⇄ 2 Hg(l) | +0,80 | |
| NO3−(aq) + 2 H+ + e− ⇄ NO2(g) + H2O | +0,80 | |
| [AuBr4]− + 3 e− ⇄ Au(s) + 4 Br− | +0,85 | |
| Hg2+ + 2 e− ⇄ Hg(l) | +0,85 | |
| MnO4− + H+ + e− ⇄ HMnO4− | +0,90 | |
| 2 Hg2+ + 2 e− ⇄ Hg22+ | +0,91 | |
| Pd2+ + 2 e− ⇄ Pd(s) | +0,915 | |
| [AuCl4]− + 3 e− ⇄ Au(s) + 4 Cl− | +0,93 | |
| MnO2(s) + 4 H+ + e− ⇄ Mn3+ + 2 H2O | +0,95 | |
| [AuBr2]− + e− ⇄ Au(s) + 2 Br− | +0,96 | |
| Br2(l) + 2 e− ⇄ 2 Br− | +1,066 | |
| Br2(aq) + 2 e− ⇄ 2 Br− | +1,0873 | |
| IO3− + 5 H+ + 4 e− ⇄ HIO(aq) + 2 H2O | +1,13 | |
| [AuCl2]− + e− ⇄ Au(s) + 2 Cl− | +1,15 | |
| HSeO4− + 3 H+ + 2 e− ⇄ H2SeO3(aq) + H2O | +1,15 | |
| Ag2O(s) + 2 H+ + 2 e− ⇄ 2 Ag(s) + H2O | +1,17 | |
| ClO3− + 2 H+ + e− ⇄ ClO2(g) + H2O | +1,18 | |
| Pt2+ + 2 e− ⇄ Pt(s) | +1,188 | |
| ClO2(g) + H+ + e− ⇄ HClO2(aq) | +1,19 | |
| 2 IO3− + 12 H+ + 10 e− ⇄ I2(s) + 6 H2O | +1,20 | |
| ClO4− + 2 H+ + 2 e− ⇄ ClO3− + H2O | +1,20 | |
| O2(g) + 4 H+ + 4 e− ⇄ 2 H2O | +1,23 | |
| MnO2(s) + 4 H+ + 2 e− ⇄ Mn2+ + 2H2O | +1,23 | |
| Tl3+ + 2 e− ⇄ Tl+ | +1,25 | |
| Cl2(g) + 2 e− ⇄ 2 Cl− | +1,36 | |
| Cr2O7− − + 14 H+ + 6 e− ⇄ 2 Cr3+ + 7 H2O | +1,33 | |
| CoO2(s) + 4 H+ + e− ⇄ Co3+ + 2 H2O | +1,42 | |
| 2 NH3OH+ + H+ + 2 e− ⇄ N2H5+ + 2 H2O | +1,42 | |
| 2 HIO(aq) + 2 H+ + 2 e− ⇄ I2(s) + 2 H2O | +1,44 | |
| Ce4+ + e− ⇄ Ce3+ | +1,44 | |
| BrO3− + 5 H+ + 4 e− ⇄ HBrO(aq) + 2 H2O | +1,45 | |
| β-PbO2(s) + 4 H+ + 2 e− ⇄ Pb2+ + 2 H2O | +1,460 | |
| α-PbO2(s) + 4 H+ + 2 e− ⇄ Pb2+ + 2 H2O | +1,468 | |
| 2 BrO3− + 12 H+ + 10 e− ⇄ Br2(l) + 6 H2O | +1,48 | |
| 2ClO3− + 12 H+ + 10 e− ⇄ Cl2(g) + 6 H2O | +1,49 | |
| MnO4− + 8 H+ + 5 e− ⇄ Mn2+ + 4 H2O | +1,51 | |
| HO2• + H+ + e− ⇄ H2O2(aq) | +1,51 | |
| Au3+ + 3 e− ⇄ Au(s) | +1,52 | |
| NiO2(s) + 4 H+ + 2 e− ⇄ Ni2+ + 2 OH− | +1,59 | |
| 2 HClO(aq) + 2 H+ + 2 e− ⇄ Cl2(g) + 2 H2O | +1,63 | |
| Ag2O3(s) + 6 H+ + 4 e− ⇄ 2 Ag+ + 3 H2O | +1,67 | |
| HClO2(aq) + 2 H+ + 2 e− ⇄ HClO(aq) + H2O | +1,67 | |
| Pb4+ + 2 e− ⇄ Pb2+ | +1,69 | |
| MnO4− + 4 H+ + 3 e− ⇄ MnO2(s) + 2 H2O | +1,70 | |
| H2O2(aq) + 2 H+ + 2 e− ⇄ 2 H2O | +1,78 | |
| AgO(s) + 2 H+ + e− ⇄ Ag+ + H2O | +1,77 | |
| Co3+ + e− ⇄ Co2+ | +1,82 | |
| Au+ + e− ⇄ Au(s) | +1,83 | |
| BrO4− + 2 H+ + 2 e− ⇄ BrO3− + H2O | +1,85 | |
| Ag2+ + e− ⇄ Ag+ | +1,98 | |
| S2O82− + 2 e− ⇄ 2 SO42− | +2,010 | |
| O3(g) + 2 H+ + 2 e− ⇄ O2(g) + H2O | +2,075 | |
| HMnO4− + 3 H+ + 2 e− ⇄ MnO2(s) + 2 H2O | +2,09 | |
| F2(g) + 2 e− ⇄ 2 F− | +2,87 | |
| F2(g) + 2 H+ + 2 e− ⇄ 2 HF(aq) | +3,05 |